Plasma metabolomics reveals disrupted response and recovery following maximal exercise in myalgic encephalomyelitis/chronic fatigue syndrome
- PMID: 35358096
- PMCID: PMC9090259
- DOI: 10.1172/jci.insight.157621
Plasma metabolomics reveals disrupted response and recovery following maximal exercise in myalgic encephalomyelitis/chronic fatigue syndrome
Abstract
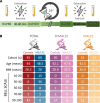
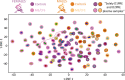
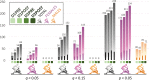
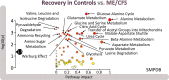
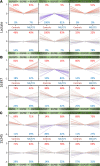
Post-exertional malaise (PEM) is a hallmark symptom of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). We monitored the evolution of 1157 plasma metabolites in 60 ME/CFS (45 female, 15 male) and 45 matched healthy control participants (30 female, 15 male) before and after 2 maximal cardiopulmonary exercise test (CPET) challenges separated by 24 hours, with the intent of provoking PEM in patients. Four time points allowed exploration of the metabolic response to maximal energy-producing capacity and the recovery pattern of participants with ME/CFS compared with the healthy control group. Baseline comparison identified several significantly different metabolites, along with an enriched percentage of yet-to-be identified compounds. Additionally, temporal measures demonstrated an increased metabolic disparity between cohorts, including unknown metabolites. The effects of exertion in the ME/CFS cohort predominantly highlighted lipid-related as well as energy-related pathways and chemical structure clusters, which were disparately affected by the first and second exercise sessions. The 24-hour recovery period was distinct in the ME/CFS cohort, with over a quarter of the identified pathways statistically different from the controls. The pathways that are uniquely different 24 hours after an exercise challenge provide clues to metabolic disruptions that lead to PEM. Numerous altered pathways were observed to depend on glutamate metabolism, a crucial component of the homeostasis of many organs in the body, including the brain.
Keywords: Amino acid metabolism; Carbohydrate metabolism; Glucose metabolism; Metabolism.
Conflict of interest statement
Figures







References
-
- Institute of Medicine of The National Academies, ed. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. National Academies Press; 2015. - PubMed
-
- Bell DS, ed. The Doctor’s Guide to Chronic Fatigue Syndrome: Understanding, Treating, and Living with CFIDS. Addison-Wesley; 1994.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

