Use of At-Home COVID-19 Tests - United States, August 23, 2021-March 12, 2022
- PMID: 35358168
- PMCID: PMC8979595
- DOI: 10.15585/mmwr.mm7113e1
Use of At-Home COVID-19 Tests - United States, August 23, 2021-March 12, 2022
Abstract
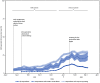
COVID-19 testing provides information regarding exposure and transmission risks, guides preventative measures (e.g., if and when to start and end isolation and quarantine), identifies opportunities for appropriate treatments, and helps assess disease prevalence (1). At-home rapid COVID-19 antigen tests (at-home tests) are a convenient and accessible alternative to laboratory-based diagnostic nucleic acid amplification tests (NAATs) for SARS-CoV-2, the virus that causes COVID-19 (2-4). With the emergence of the SARS-CoV-2 B.1.617.2 (Delta) and B.1.1.529 (Omicron) variants in 2021, demand for at-home tests increased† (5). At-home tests are commonly used for school- or employer-mandated testing and for confirmation of SARS-CoV-2 infection in a COVID-19-like illness or following exposure (6). Mandated COVID-19 reporting requirements omit at-home tests, and there are no standard processes for test takers or manufacturers to share results with appropriate health officials (2). Therefore, with increased COVID-19 at-home test use, laboratory-based reporting systems might increasingly underreport the actual incidence of infection. Data from a cross-sectional, nonprobability-based online survey (August 23, 2021-March 12, 2022) of U.S. adults aged ≥18 years were used to estimate self-reported at-home test use over time, and by demographic characteristics, geography, symptoms/syndromes, and reasons for testing. From the Delta-predominant period (August 23-December 11, 2021) to the Omicron-predominant period (December 19, 2021-March 12, 2022)§ (7), at-home test use among respondents with self-reported COVID-19-like illness¶ more than tripled from 5.7% to 20.1%. The two most commonly reported reasons for testing among persons who used an at-home test were COVID-19 exposure (39.4%) and COVID-19-like symptoms (28.9%). At-home test use differed by race (e.g., self-identified as White [5.9%] versus self-identified as Black [2.8%]), age (adults aged 30-39 years [6.4%] versus adults aged ≥75 years [3.6%]), household income (>$150,000 [9.5%] versus $50,000-$74,999 [4.7%]), education (postgraduate degree [8.4%] versus high school or less [3.5%]), and geography (New England division [9.6%] versus West South Central division [3.7%]). COVID-19 testing, including at-home tests, along with prevention measures, such as quarantine and isolation when warranted, wearing a well-fitted mask when recommended after a positive test or known exposure, and staying up to date with vaccination,** can help reduce the spread of COVID-19. Further, providing reliable and low-cost or free at-home test kits to underserved populations with otherwise limited access to COVID-19 testing could assist with continued prevention efforts.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Christina M. Astley reports grants from Flu Lab and from the National Institutes of Health and the National Institute of Diabetes and Digestive and Kidney Diseases during conduct of the study. John S. Brownstein, Autumn Gertz, Benjamin Rader, Kara Sewalk, and Tanner J. Varrelman report grants from Flu Lab during conduct of the study. No other potential conflicts of interest were disclosed.
Figures
References
-
- Food and Drug Administration. In vitro diagnostics EUAs - antigen diagnostic tests for SARS-CoV-2. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2022. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-em...
-
- CDC. COVID-19. Nucleic acid amplification tests (NAATs). Atlanta, GA: US Department of Health and Human Services, CDC: 2020. https://www.cdc.gov/coronavirus/2019-ncov/lab/naats.html
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

