Exploring a peptide nucleic acid-based antisense approach for CD5 targeting in chronic lymphocytic leukemia
- PMID: 35358273
- PMCID: PMC8970396
- DOI: 10.1371/journal.pone.0266090
Exploring a peptide nucleic acid-based antisense approach for CD5 targeting in chronic lymphocytic leukemia
Abstract
We herein report an innovative antisense approach based on Peptide Nucleic Acids (PNAs) to down-modulate CD5 expression levels in chronic lymphocytic leukemia (CLL). Using bioinformatics tools, we selected a 12-mer tract of the CD5 mRNA as the molecular target and synthesized the complementary and control PNA strands bearing a serine phosphate dipeptide tail to enhance their water solubility and bioavailability. The specific recognition of the 12-mer DNA strand, corresponding to the target mRNA sequence by the complementary PNA strand, was confirmed by non-denaturing polyacrylamide gel electrophoresis, thermal difference spectroscopy, circular dichroism (CD), and CD melting studies. Cytofluorimetric assays and real-time PCR analysis demonstrated the downregulation of CD5 expression due to incubation with the anti-CD5 PNA at RNA and protein levels in Jurkat cell line and peripheral blood mononuclear cells from B-CLL patients. Interestingly, we also observed that transfection with the anti-CD5 PNA increases apoptotic response induced by fludarabine in B-CLL cells. The herein reported results suggest that PNAs could represent a potential candidate for the development of antisense therapeutic agents in CLL.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

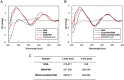
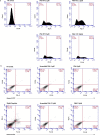
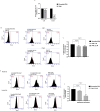
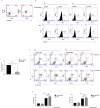
References
-
- Starostka D, Kriegova E, Kudelka M, Mikula P, Zehnalova S, Radvansky M, et al.. Quantitative assessment of informative immunophenotypic markers increases the diagnostic value of immunophenotyping in mature CD5-positive B-cell neoplasms. Cytom Part B—Clin Cytom. 2018;94: 576–587. doi: 10.1002/cyto.b.21607 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

