Bevacizumab in High-Risk Corneal Transplantation: A Pilot Multicenter Prospective Randomized Control Trial
- PMID: 35358592
- PMCID: PMC10742165
- DOI: 10.1016/j.ophtha.2022.03.024
Bevacizumab in High-Risk Corneal Transplantation: A Pilot Multicenter Prospective Randomized Control Trial
Erratum in
-
Corrigendum.Ophthalmology. 2022 Nov;129(11):1334. doi: 10.1016/j.ophtha.2022.08.006. Ophthalmology. 2022. PMID: 36272763 No abstract available.
Abstract
Purpose: To determine the efficacy of local (subconjunctival and topical) bevacizumab (Avastin) treatment in patients undergoing vascularized high-risk corneal transplantation.
Design: Pilot, prospective, randomized, double-blind, placebo-controlled clinical trial conducted at 5 clinical centers in the United States, India, and Brazil.
Participants: Patients aged > 18 years undergoing high-risk penetrating keratoplasty, defined as corneal neovascularization (NV) in 1 or more quadrants ≥2 mm from the limbus or extension of corneal NV to the graft-host junction in a previously failed graft.
Methods: Patients were randomized to receive subconjunctival bevacizumab (2.5 mg/0.1 ml) or placebo at the time of surgery, followed by topical bevacizumab (10 mg/ml) or topical placebo, administered 4 times per day for 4 weeks.
Main outcome measure: The 52-week endothelial immune rejection rate.
Results: Ninety-two patients were randomized to receive bevacizumab (n = 48) or control (n = 44). The 52-week endothelial rejection rate was 10% in the bevacizumab group and 19% in the control group (P = 0.20). Post hoc, extended follow-up at the lead study site showed an endothelial rejection rate of 3% in the bevacizumab group and 38% in the control group (P = 0.003). Treatment with bevacizumab was found to have a hazard ratio of 0.15 (95% confidence interval, 0.03-0.65, P = 0.01) in a post hoc Cox regression analysis.
Conclusions: In patients undergoing vascularized high-risk corneal transplantation, there was no statistically significant difference in the rate of endothelial rejection at 1 year in the bevacizumab treatment group compared with the control group. This study may have been underpowered to detect a difference between treatment groups, and taken together, our data suggest that, in the current trial design, bevacizumab has a positive but not (yet) significant effect on endothelial rejection.
Keywords: Angiogenesis; Corneal transplantation; Neovascularization; Penetrating keratoplasty; Vascular endothelial growth factor.
Copyright © 2022 American Academy of Ophthalmology. Published by Elsevier Inc. All rights reserved.
Figures





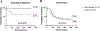

References
-
- WHO. Blindness and Vision Impairment. World Health Organization; 2021. https://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-im.... Accessed August 10, 2021.
-
- Eye Bank Association of American. 2019 Eye Banking Statistical Report. Washington, DC: Eye Bank Association of America; 2019.
-
- Alio JL, Montesel A, Sayyad F, et al. Corneal graft failure: an update. Br J Ophthalmol. 2021;105:1049–1058. - PubMed
-
- The Collaborative Corneal Transplantation Studies (CCTS). Effectiveness of histocompatibility matching in high-risk corneal transplantation. The Collaborative Corneal Transplantation Studies Research Group. Arch Ophthalmol. 1992;110:1392–1403. - PubMed
-
- Williams KA, Esterman AJ, Bartlett C, et al. How effective is penetrating corneal transplantation? Factors influencing long-term outcome in multivariate analysis. Transplantation. 2006;81:896–901. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

