Lung targeted liposomes for treating ARDS
- PMID: 35358610
- PMCID: PMC8958843
- DOI: 10.1016/j.jconrel.2022.03.028
Lung targeted liposomes for treating ARDS
Abstract
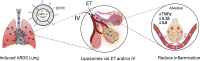
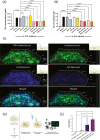
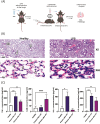
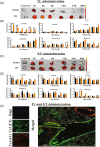
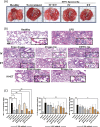
Acute Respiratory Distress Syndrome (ARDS), associated with Covid-19 infections, is characterized by diffuse lung damage, inflammation and alveolar collapse that impairs gas exchange, leading to hypoxemia and patient' mortality rates above 40%. Here, we describe the development and assessment of 100-nm liposomes that are tailored for pulmonary delivery for treating ARDS, as a model for lung diseases. The liposomal lipid composition (primarily DPPC) was optimized to mimic the lung surfactant composition, and the drug loading process of both methylprednisolone (MPS), a steroid, and N-acetyl cysteine (NAC), a mucolytic agent, reached an encapsulation efficiency of 98% and 92%, respectively. In vitro, treating lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages with the liposomes decreased TNFα and nitric oxide (NO) secretion, while NAC increased the penetration of nanoparticles through the mucus. In vivo, we used LPS-induced lung inflammation model to assess the accumulation and therapeutic efficacy of the liposomes in C57BL/6 mice, either by intravenous (IV), endotracheal (ET) or IV plus ET nanoparticles administrations. Using both administration methods, liposomes exhibited an increased accumulation profile in the inflamed lungs over 48 h. Interestingly, while IV-administrated liposomes distributed widely throughout the lung, ET liposomes were present in lungs parenchyma but were not detected at some distal regions of the lungs, possibly due to imperfect airflow regimes. Twenty hours after the different treatments, lungs were assessed for markers of inflammation. We found that the nanoparticle treatment had a superior therapeutic effect compared to free drugs in treating ARDS, reducing inflammation and TNFα, IL-6 and IL-1β cytokine secretion in bronchoalveolar lavage (BAL), and that the combined treatment, delivering nanoparticles IV and ET simultaneously, had the best outcome of all treatments. Interestingly, also the DPPC lipid component alone played a therapeutic role in reducing inflammatory markers in the lungs. Collectively, we show that therapeutic nanoparticles accumulate in inflamed lungs holding potential for treating lung disorders. SIGNIFICANCE: In this study we compare intravenous versus intratracheal delivery of nanoparticles for treating lung disorders, specifically, acute respiratory distress syndrome (ARDS). By co-loading two medications into lipid nanoparticles, we were able to reduce both inflammation and mucus secretion in the inflamed lungs. Both modes of delivery resulted in high nanoparticle accumulation in the lungs, intravenously administered nanoparticles reached lung endothelial while endotracheal delivery reached lung epithelial. Combining both delivery approaches simultaneously provided the best ARDS treatment outcome.
Keywords: ARDS; COPD; Covid-19; Liposome; Lung inflammation; Mucus; Nanotechnology; Pulmonary.
Copyright © 2022 Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Therapeutic effects of tea polyphenol-loaded nanoparticles coated with platelet membranes on LPS-induced lung injury.Biomater Sci. 2023 Sep 12;11(18):6223-6235. doi: 10.1039/d3bm00802a. Biomater Sci. 2023. PMID: 37529873
-
Delivery of miR-26a-5p by Subcutaneous Adipose Tissue-Derived Extracellular Vesicles Alleviates Acute Lung Injury in Mice Through CHUK/NF-κB Pathway.Int J Nanomedicine. 2025 May 10;20:6001-6021. doi: 10.2147/IJN.S514623. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40370804 Free PMC article.
-
Repurposing Disulfiram to Combat Acute Respiratory Distress Syndrome with Targeted Delivery by LET-Functionalized Nanoplatforms.ACS Appl Mater Interfaces. 2024 Mar 13;16(10):12244-12262. doi: 10.1021/acsami.3c17659. Epub 2024 Feb 29. ACS Appl Mater Interfaces. 2024. PMID: 38421312
-
Nanomedicine for acute respiratory distress syndrome: The latest application, targeting strategy, and rational design.Acta Pharm Sin B. 2021 Oct;11(10):3060-3091. doi: 10.1016/j.apsb.2021.04.023. Epub 2021 May 7. Acta Pharm Sin B. 2021. PMID: 33977080 Free PMC article. Review.
-
Pulmonary drug delivery devices and nanosystems as potential treatment strategies for acute respiratory distress syndrome (ARDS).Int J Pharm. 2024 May 25;657:124182. doi: 10.1016/j.ijpharm.2024.124182. Epub 2024 Apr 30. Int J Pharm. 2024. PMID: 38697584 Review.
Cited by
-
Discovery, validation, and prodrug design of an ACE2 activator for treating bacterial infection-induced lung inflammation.J Control Release. 2023 Dec;364:1-11. doi: 10.1016/j.jconrel.2023.10.025. Epub 2023 Oct 21. J Control Release. 2023. PMID: 37858626 Free PMC article.
-
Effects of Surface Charge of Inhaled Liposomes on Drug Efficacy and Biocompatibility.Pharmaceutics. 2025 Mar 3;17(3):329. doi: 10.3390/pharmaceutics17030329. Pharmaceutics. 2025. PMID: 40142994 Free PMC article.
-
Translational medicine for acute lung injury.J Transl Med. 2024 Jan 5;22(1):25. doi: 10.1186/s12967-023-04828-7. J Transl Med. 2024. PMID: 38183140 Free PMC article. Review.
-
Research Progress on Liposome Pulmonary Delivery of Mycobacterium tuberculosis Nucleic Acid Vaccine and Its Mechanism of Action.J Aerosol Med Pulm Drug Deliv. 2024 Oct;37(5):284-298. doi: 10.1089/jamp.2023.0025. Epub 2024 Apr 26. J Aerosol Med Pulm Drug Deliv. 2024. PMID: 38669118 Review.
-
[Research advances of liposomes and exosomes in drug delivery and biomarker screening].Se Pu. 2025 May;43(5):472-486. doi: 10.3724/SP.J.1123.2024.08012. Se Pu. 2025. PMID: 40331611 Free PMC article. Review. Chinese.
References
-
- Mark D Siege . UpToDate; 2022. Acute Respiratory Distress Syndrome: Epidemiology, Pathophysiology, Pathology, and Etiology in Adults.
-
- Lilah Lopez T.N., Weber Graham, Kleimola Katlyn, Bereda Megan, Liu Yiling, Accorsi Emma K., Skates Steven J., Santa Maria John P., Jr., Smith Kendal R., Kalinich Mark. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in the staff of a public school system in the midwestern United States. PLoS One. 2021;16 doi: 10.1371/journal.pone.0243676. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

