An SCD1-dependent mechanoresponsive pathway promotes HCC invasion and metastasis through lipid metabolic reprogramming
- PMID: 35358687
- PMCID: PMC9263248
- DOI: 10.1016/j.ymthe.2022.03.015
An SCD1-dependent mechanoresponsive pathway promotes HCC invasion and metastasis through lipid metabolic reprogramming
Abstract
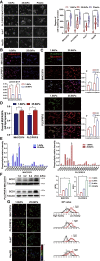
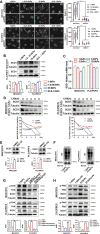
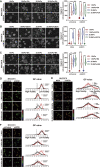
Matrix stiffness promotes hepatocellular carcinoma (HCC) metastasis. This study examined the contribution of lipid metabolic reprogramming to matrix stiffness-induced HCC metastasis. HCC cells were cultured on mechanically tunable polyacrylamide gels and subjected to lipidomic analysis. The key enzyme that responded to matrix stiffness and regulated lipid metabolism was identified. The comparative lipidomic screening revealed that stearoyl-CoA desaturase 1 (SCD1) is a mechanoresponsive enzyme that reprogrammed HCC cell lipid metabolism. The genetic and pharmacological inhibition of SCD1 expression/activity altered the cellular lipid composition, which in turn impaired plasma membrane fluidity and inhibited in vitro invasive motility of HCC cells in response to high matrix stiffness. Knockdown of SCD1 suppressed HCC invasion and metastasis in vivo. Conversely, the overexpression of SCD1 or exogenous administration of its product oleic acid augmented plasma membrane fluidity and rescued in vitro invasive migration in HCC cells cultured on soft substrates, mimicking the effects imposed by high matrix stiffness. In human HCC tissues, collagen content, a marker of increasing matrix stiffness, and increased expression of SCD1 together predicted poor survival of HCC patients. An SCD1-dependent mechanoresponsive pathway that responds to increasing matrix stiffness in the tumor microenvironment promotes HCC invasion and metastasis through lipid metabolic reprogramming.
Keywords: extracellular matrix stiffness; hepatocellular carcinoma; lipid metabolism; membrane fluidity.
Copyright © 2022 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest The authors declare no competing interests.
Figures




References
-
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. - PubMed
-
- Ishizawa T., Hasegawa K., Aoki T., Takahashi M., Inoue Y., Sano K., et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908–1916. - PubMed
-
- Ye Q.H., Qin L.X., Forgues M., He P., Kim J.W., Peng A.C., et al. Predicting hepatitis B virus-positive metastatic hepatocellular carcinomas using gene expression profiling and supervised machine learning. Nat. Med. 2003;9:416–423. - PubMed
-
- Tang Z.Y., Ye S.L., Liu Y.K., Qin L.X., Sun H.C., Ye Q.H., et al. A decade's studies on metastasis of hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2004;130:187–196. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

