Long-term outcomes of dexamethasone 12 mg versus 6 mg in patients with COVID-19 and severe hypoxaemia
- PMID: 35359168
- PMCID: PMC8970069
- DOI: 10.1007/s00134-022-06677-2
Long-term outcomes of dexamethasone 12 mg versus 6 mg in patients with COVID-19 and severe hypoxaemia
Abstract
Purpose: We assessed long-term outcomes of dexamethasone 12 mg versus 6 mg given daily for up to 10 days in patients with coronavirus disease 2019 (COVID-19) and severe hypoxaemia.
Methods: We assessed 180-day mortality and health-related quality of life (HRQoL) using EuroQoL (EQ)-5D-5L index values and EQ visual analogue scale (VAS) in the international, stratified, blinded COVID STEROID 2 trial, which randomised 1000 adults with confirmed COVID-19 receiving at least 10 L/min of oxygen or mechanical ventilation in 26 hospitals in Europe and India. In the HRQoL analyses, higher values indicated better outcomes, and deceased patients were given a score of zero.
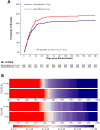
Results: We obtained vital status at 180 days for 963 of 982 patients (98.1%) in the intention-to-treat population, EQ-5D-5L index value data for 922 (93.9%) and EQ VAS data for 924 (94.1%). At 180 days, 164 of 486 patients (33.7%) had died in the 12 mg group versus 184 of 477 (38.6%) in the 6 mg group [adjusted risk difference - 4.3%; 99% confidence interval (CI) - 11.7-3.0; relative risk 0.89; 0.72-1.09; P = 0.13]. The adjusted mean differences between the 12 mg and the 6 mg groups in EQ-5D-5L index values were 0.06 (99% CI - 0.01 to 0.12; P = 0.10) and in EQ VAS scores 4 (- 3 to 10; P = 0.22).
Conclusion: Among patients with COVID-19 and severe hypoxaemia, dexamethasone 12 mg compared with 6 mg did not result in statistically significant improvements in mortality or HRQoL at 180 days, but the results were most compatible with benefit from the higher dose.
Trial registration: ClinicalTrials.gov NCT04509973.
Keywords: COVID-19; Corticosteroids; Critical illness; Hypoxaemia; Mortality; Quality of life.
© 2022. Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
AG, MBNK, MWM, GKV, TSM, MHM and AP are affiliated with the Dept. of Intensive Care, Rigshospitalet, which has received grants for research from Pfizer, Fresenius Kabi, AM Pharma, and Sygeforsikringen ‘danmark’ outside the submitted work. MH has participated in advisory boards for AstraZeneca, GSK, Gilead, MSD, Roche and Sobi and received speaker’s honoraria from GSK and Gilead. TB reports grants from Novo Nordisk Foundation, grants from Simonsen Foundation, grants and personal fees from GSK, grants and personal fees from Pfizer, personal fees from Astra Zeneca, personal fees from Janssen, personal fees from Boehringer Ingelheim, grants and personal fees from Gilead, personal fees from MSD, grants from Lundbeck Foundation, grants from Kai Hansen Foundation, personal fees from Pentabase ApS, grants from Erik and Susanna Olesen’s Charitable Fund, outside the submitted work. SMJ and LC are affiliated with Inselspital, Bern University Hospital, which has received grants from Edwards Lifesciences Services GmbH, Phagenesis Limited, and Nestlé outside the submitted work. JVD has received personal fees (paid to his institution) from Edwards India outside the submitted work. VJ has received grant funding from GSK, Baxter Healthcare, and Biocon and honoraria from Boehringer Ingelheim, Astra Zeneca, Baxter Healthcare, Bayer, NephroPlus and Zydus Cadilla, under the policy of all monies being paid to the organization.
Figures

References
-
- Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, Annane D, Azevedo LCP, Berwanger O, Cavalcanti AB, Dequin PF, Du B, Emberson J, Fisher D, Giraudeau B, Gordon AC, Granholm A, Green C, Haynes R, Heming N, Higgins JPT, Horby P, Juni P, Landray MJ, Le Gouge A, Leclerc M, Lim WS, Machado FR, McArthur C, Meziani F, Moller MH, Perner A, Petersen MW, Savovic J, Tomazini B, Veiga VC, Webb S, Marshall JC. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. - DOI - PMC - PubMed
-
- Rochwerg B, Siemieniuk RA, Agoritsas T, Lamontagne F, Askie L, Lytvyn L, Agarwal A, Leo YS, Macdonald H, Zeng L, Amin W, Burhan E, Bausch FJ, Calfee CS, Cecconi M, Chanda D, Du B, Geduld H, Gee P, Harley N, Hashimi M, Hunt B, Kabra SK, Kanda S, Kawano-Dourado L, Kim YJ, Kissoon N, Kwizera A, Mahaka I, Manai H, Mino G, Nsutebu E, Pshenichnaya N, Qadir N, Sabzwari S, Sarin R, Shankar-Hari M, Sharland M, Shen Y, Ranganathan SS, Souza JP, Stegemann M, De Sutter A, Ugarte S, Venkatapuram S, Dat VQ, Vuyiseka D, Wijewickrama A, Maguire B, Zeraatkar D, Bartoszko JJ, Ge L, Brignardello-Petersen R, Owen A, Guyatt G, Diaz J, Jacobs M, Vandvik PO. A living WHO guideline on drugs for COVID-19. BMJ. 2020;370:m3379. - PubMed
-
- Munch MW, Myatra SN, Vijayaraghavan BKT, Saseedharan S, Benfield T, Wahlin RR, Rasmussen BS, Andreasen AS, Poulsen LM, Cioccari L, Khan MS, Kapadia F, Divatia JV, Brochner AC, Bestle MH, Helleberg M, Michelsen J, Padmanaban A, Bose N, Moller A, Borawake K, Kristiansen KT, Shukla U, Chew MS, Dixit S, Ulrik CS, Amin PR, Chawla R, Wamberg CA, Shah MS, Darfelt IS, Jorgensen VL, Smitt M, Granholm A, Kjaer MN, Moller MH, Meyhoff TS, Vesterlund GK, Hammond NE, Micallef S, Bassi A, John O, Jha A, Cronhjort M, Jakob SM, Gluud C, Lange T, Kadam V, Marcussen KV, Hollenberg J, Hedman A, Nielsen H, Schjorring OL, Jensen MQ, Leistner JW, Jonassen TB, Kristensen CM, Clapp EC, Hjortso CJS, Jensen TS, Halstad LS, Bak ERB, Zaabalawi R, Metcalf-Clausen M, Abdi S, Hatley EV, Aksnes TS, Gleipner-Andersen E, Alarcon AF, Yamin G, Heymowski A, Berggren A, La Cour K, Weihe S, Pind AH, Engstrom J, Jha V, Venkatesh B, Perner A. Effect of 12 mg vs 6 mg of dexamethasone on the number of days alive without life support in adults with COVID-19 and Severe hypoxemia: the COVID STEROID 2 randomized trial. JAMA. 2021;326:1807–1817. doi: 10.1001/jama.2021.18295. - DOI - PMC - PubMed
-
- Granholm A, Munch MW, Myatra SN, Vijayaraghavan BKT, Cronhjort M, Wahlin RR, Jakob SM, Cioccari L, Kjaer MN, Vesterlund GK, Meyhoff TS, Helleberg M, Moller MH, Benfield T, Venkatesh B, Hammond NE, Micallef S, Bassi A, John O, Jha V, Kristiansen KT, Ulrik CS, Jorgensen VL, Smitt M, Bestle MH, Andreasen AS, Poulsen LM, Rasmussen BS, Brochner AC, Strom T, Moller A, Khan MS, Padmanaban A, Divatia JV, Saseedharan S, Borawake K, Kapadia F, Dixit S, Chawla R, Shukla U, Amin P, Chew MS, Wamberg CA, Gluud C, Lange T, Perner A. Dexamethasone 12 mg versus 6 mg for patients with COVID-19 and severe hypoxaemia: a pre-planned, secondary Bayesian analysis of the COVID STEROID 2 trial. Intensive Care Med. 2022;48:45–55. doi: 10.1007/s00134-021-06573-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

