Artificial Intelligence in Cervical Cancer Screening and Diagnosis
- PMID: 35359358
- PMCID: PMC8963491
- DOI: 10.3389/fonc.2022.851367
Artificial Intelligence in Cervical Cancer Screening and Diagnosis
Abstract
Cervical cancer remains a leading cause of cancer death in women, seriously threatening their physical and mental health. It is an easily preventable cancer with early screening and diagnosis. Although technical advancements have significantly improved the early diagnosis of cervical cancer, accurate diagnosis remains difficult owing to various factors. In recent years, artificial intelligence (AI)-based medical diagnostic applications have been on the rise and have excellent applicability in the screening and diagnosis of cervical cancer. Their benefits include reduced time consumption, reduced need for professional and technical personnel, and no bias owing to subjective factors. We, thus, aimed to discuss how AI can be used in cervical cancer screening and diagnosis, particularly to improve the accuracy of early diagnosis. The application and challenges of using AI in the diagnosis and treatment of cervical cancer are also discussed.
Keywords: artificial intelligence; cervical cancer; cervical intraepithelial neoplasia (CIN); colposcopy; cytology; deep learning; early screening and diagnosis.
Copyright © 2022 Hou, Shen, Zhou, Li, Wang and Ma.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

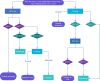

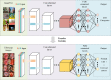
References
-
- Brisson M, Kim JJ, Canfell K, Drolet M, Gingras G, Burger EA, et al. Impact of HPV Vaccination and Cervical Screening on Cervical Cancer Elimination: A Comparative Modelling Analysis in 78 Low-Income and Lower-Middle-Income Countries. Lancet (2020) 395(10224):575–90. doi: 10.1016/S0140-6736(20)30068-4 - DOI - PMC - PubMed
-
- Simms KT, Steinberg J, Caruana M, Smith MA, Lew JB, Soerjomataram I, et al. Impact of Scaled Up Human Papillomavirus Vaccination and Cervical Screening and the Potential for Global Elimination of Cervical Cancer in 181 Countries, 2020-99: A Modelling Study. Lancet Oncol (2019) 20(3):394–407. doi: 10.1016/S1470-2045(18)30836-2 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

