Ras and Rab Interactor 3: From Cellular Mechanisms to Human Diseases
- PMID: 35359443
- PMCID: PMC8963869
- DOI: 10.3389/fcell.2022.824961
Ras and Rab Interactor 3: From Cellular Mechanisms to Human Diseases
Abstract
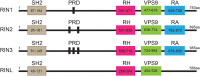
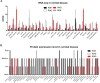
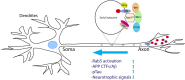
Ras and Rab interactor 3 (RIN3) functions as a Guanine nucleotide Exchange Factor (GEF) for some members of the Rab family of small GTPase. By promoting the activation of Rab5, RIN3 plays an important role in regulating endocytosis and endocytic trafficking. In addition, RIN3 activates Ras, another small GTPase, that controls multiple signaling pathways to regulate cellular function. Increasing evidence suggests that dysregulation of RIN3 activity may contribute to the pathogenesis of several disease conditions ranging from Paget's Disease of the Bone (PDB), Alzheimer's Disease (AD), Chronic Obstructive Pulmonary Disease (COPD) and to obesity. Recent genome-wide association studies (GWAS) identified variants in the RIN3 gene to be linked with these disease conditions. Interestingly, some variants appear to be missense mutations in the functional domains of the RIN3 protein while most variants are located in the noncoding regions of the RIN3 gene, potentially altering its gene expression. However, neither the protein structure of RIN3 nor its exact function(s) (except for its GEF activity) has been fully defined. Furthermore, how the polymorphisms/variants contribute to disease pathogenesis remain to be understood. Herein, we examine, and review published studies in an attempt to provide a better understanding of the physiological function of RIN3; More importantly, we construct a framework linking the polymorphisms/variants of RIN3 to altered cell signaling and endocytic traffic, and to potential disease mechanism(s).
Keywords: Alzheimer’s disease; RIN3; Rab5; endocytosis; trafficking.
Copyright © 2022 Shen, Murphy, Xu, Hu, Ding and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources

