Potential of Colostrum-Derived Exosomes for Promoting Hair Regeneration Through the Transition From Telogen to Anagen Phase
- PMID: 35359449
- PMCID: PMC8960251
- DOI: 10.3389/fcell.2022.815205
Potential of Colostrum-Derived Exosomes for Promoting Hair Regeneration Through the Transition From Telogen to Anagen Phase
Abstract
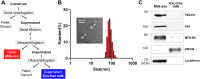
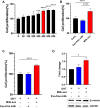
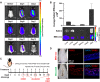
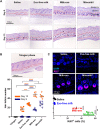
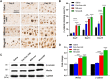
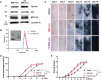
Human hair dermal papillary (DP) cells comprising mesenchymal stem cells in hair follicles contribute critically to hair growth and cycle regulation. The transition of hair follicles from telogen to anagen phase is the key to regulating hair growth, which relies heavily on the activation of DP cells. In this paper, we suggested exosomes derived from bovine colostrum (milk exosomes, Milk-exo) as a new effective non-surgical therapy for hair loss. Results showed that Milk-exo promoted the proliferation of hair DP cells and rescued dihydrotestosterone (DHT, androgen hormones)-induced arrest of follicle development. Milk-exo also induced dorsal hair re-growth in mice at the level comparable to minoxidil treatment, without associated adverse effects such as skin rashes. Our data demonstrated that Milk-exo accelerated the hair cycle transition from telogen to anagen phase by activating the Wnt/β-catenin pathway. Interestingly, Milk-exo has been found to stably retain its original properties and efficacy for hair regeneration after freeze-drying and resuspension, which is considered critical to use it as a raw material applied in different types of alopecia medicines and treatments. Overall, this study highlights a great potential of an exosome from colostrum as a therapeutic modality for hair loss.
Keywords: colostrum; dermal papilla; exosome; hair growth; lactoferrin.
Copyright © 2022 Kim, Jang, Kim, Jang, Cho, Han, Song, Kim and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources

