Functional Nano-Objects by Electrostatic Self-Assembly: Structure, Switching, and Photocatalysis
- PMID: 35359487
- PMCID: PMC8961288
- DOI: 10.3389/fchem.2021.779360
Functional Nano-Objects by Electrostatic Self-Assembly: Structure, Switching, and Photocatalysis
Abstract
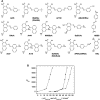
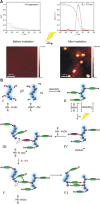
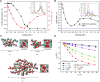
The design of functional nano-objects by electrostatic self-assembly in solution signifies an emerging field with great potential. More specifically, the targeted combination of electrostatic interaction with other effects and interactions, such as the positioning of charges on stiff building blocks, the use of additional amphiphilic, π-π stacking building blocks, or polyelectrolytes with certain architectures, have recently promulgated electrostatic self-assembly to a principle for versatile defined structure formation. A large variety of architectures from spheres over rods and hollow spheres to networks in the size range of a few tenths to a few hundred nanometers can be formed. This review discusses the state-of-the-art of different approaches of nano-object formation by electrostatic self-assembly against the backdrop of corresponding solid materials and assemblies formed by other non-covalent interactions. In this regard, particularly promising is the facile formation of triggerable structures, i.e. size and shape switching through light, as well as the use of electrostatically assembled nano-objects for improved photocatalysis and the possible solar energy conversion in the future. Lately, this new field is eliciting an increasing amount of understanding; insights and limitations thereof are addressed in this article. Special emphasis is placed on the interconnection of molecular building block structures and the resulting nanoscale architecture via the key of thermodynamics.
Keywords: nanostructures; organic-inorganic hybrids; photocatalysis; self-assembly; stimuli-responsiveness; structure analysis; supramolecular chemistry; thermodynamics.
Copyright © 2022 Krieger, Zika and Gröhn.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

















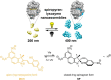




References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

