Hyperphosphorylated Human Tau Accumulates at the Synapse, Localizing on Synaptic Mitochondrial Outer Membranes and Disrupting Respiration in a Mouse Model of Tauopathy
- PMID: 35359570
- PMCID: PMC8960727
- DOI: 10.3389/fnmol.2022.852368
Hyperphosphorylated Human Tau Accumulates at the Synapse, Localizing on Synaptic Mitochondrial Outer Membranes and Disrupting Respiration in a Mouse Model of Tauopathy
Abstract
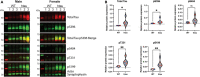
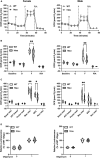
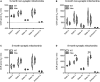
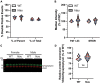
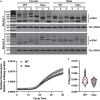
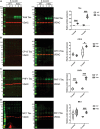
Neurogenerative disorders, such as Alzheimer's disease (AD), represent a growing public health challenge in aging societies. Tauopathies, a subset of neurodegenerative disorders that includes AD, are characterized by accumulation of fibrillar and hyperphosphorylated forms of microtubule-associated protein tau with coincident mitochondrial abnormalities and neuronal dysfunction. Although, in vitro, tau impairs axonal transport altering mitochondrial distribution, clear in vivo mechanisms associating tau and mitochondrial dysfunction remain obscure. Herein, we investigated the effects of human tau on brain mitochondria in vivo using transgenic htau mice at ages preceding and coinciding with onset of tauopathy. Subcellular proteomics combined with bioenergetic assessment revealed pathologic forms of tau preferentially associate with synaptic over non-synaptic mitochondria coinciding with changes in bioenergetics, reminiscent of an aged synaptic mitochondrial phenotype in wild-type mice. While mitochondrial content was unaltered, mitochondrial maximal respiration was impaired in synaptosomes from htau mice. Further, mitochondria-associated tau was determined to be outer membrane-associated using the trypsin protection assay and carbonate extraction. These findings reveal non-mutant human tau accumulation at the synapse has deleterious effects on mitochondria, which likely contributes to synaptic dysfunction observed in the context of tauopathy.
Keywords: Alzheimer’s disease; aging; bioenergetics; phosphorylation; proteomics; synaptic mitochondria; tau; tauopathy.
Copyright © 2022 Trease, George, Roland, Lichter, Emanuel, Totusek, Fox and Stauch.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

