Synergistic Effect of Quercetin Magnetite Nanoparticles and Targeted Radiotherapy in Treatment of Breast Cancer
- PMID: 35359610
- PMCID: PMC8961357
- DOI: 10.1177/11782234221086728
Synergistic Effect of Quercetin Magnetite Nanoparticles and Targeted Radiotherapy in Treatment of Breast Cancer
Abstract
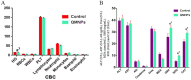
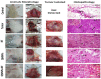
Quercetin is a potent cancer therapeutic agent present in fruits and vegetables. The pharmaceutical uses of quercetin are limited due to many problems associated with low solubility, bioavailability, permeability, and instability. In addition, the high doses of quercetin show toxic effects in clinical and experimental studies. Therefore, a new strategy is warranted to overcome these problems without the use of toxic doses. The iron oxide nanoparticles can be used as a drug delivery system. This study aimed to prepare quercetin-conjugated magnetite nanoparticles (QMNPs) using biological simple nanoprecipitation and mediated by fungus Aspergillus oryzae. Also, we initiated in vitro and in vivo studies to determine whether QMNPs might sensitize breast cancer to radiotherapy treatment. The structural, morphological, and magnetic properties of the prepared nanoparticles were studied. The results indicated that QMNPs were spherical in shape and 40 nm in diameter. The in vitro studies showed that the incubation of MCF-7, HePG-2, and A459 cancer cells with QMNPs for 24 h effectively inhibited the growth of cancer cell lines in a concentration-dependent manner with IC50 values of 11, 77.5, and104 nmol/mL, respectively. The combination of QMNPs with irradiation (IR) potently blocked MCF-7 cancer cell proliferation and showed significant changes in the morphology of these cells as observed by bright-field inverted light microscopy. Focusing on the long-term toxicity of QMNPs (20 ml/kg), the assessment of hematological, hepatic, and renal markers indicated no toxic effect. Besides, QMNPs inhibited tumor growth and potently enhanced the lateral radiotherapy treatment in N-methyl-N-nitrosourea (MNU)-induced breast cancer in female white albino rats. These anticancer and radiosensitizing activities were ascribed to cytotoxicity, cell cycle arrest, immunomodulation, and efficiency through induction of apoptosis. In a conclusion, these observations suggest that the QMNPs combined with LRT could act as a potential targeted therapy in breast cancer.
Keywords: Nanoparticles; apoptosis; breast cancer; magnetic oxide; methyl-N-nitrosourea; quercetin; radiotherapy.
© The Author(s) 2022.
Conflict of interest statement
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures





Similar articles
-
Quercetin conjugated superparamagnetic magnetite nanoparticles for in-vitro analysis of breast cancer cell lines for chemotherapy applications.J Colloid Interface Sci. 2014 Dec 15;436:234-42. doi: 10.1016/j.jcis.2014.08.064. Epub 2014 Sep 16. J Colloid Interface Sci. 2014. PMID: 25278361
-
Effects of Quercetin-Loaded Nanoparticles on MCF-7 Human Breast Cancer Cells.Medicina (Kaunas). 2019 Apr 22;55(4):114. doi: 10.3390/medicina55040114. Medicina (Kaunas). 2019. PMID: 31013662 Free PMC article.
-
Quercetin conjugated with silica nanoparticles inhibits tumor growth in MCF-7 breast cancer cell lines.Biochem Biophys Res Commun. 2018 Jun 12;500(4):860-865. doi: 10.1016/j.bbrc.2018.04.174. Epub 2018 Apr 26. Biochem Biophys Res Commun. 2018. PMID: 29698680
-
Quercetin Loaded Nanoparticles in Targeting Cancer: Recent Development.Anticancer Agents Med Chem. 2019;19(13):1560-1576. doi: 10.2174/1871520619666190705150214. Anticancer Agents Med Chem. 2019. PMID: 31284873 Review.
-
Quercetin nanoformulations: a promising strategy for tumor therapy.Food Funct. 2021 Aug 2;12(15):6664-6681. doi: 10.1039/d1fo00851j. Food Funct. 2021. PMID: 34152346 Review.
Cited by
-
Quercetin Alleviates Lipopolysaccharide-Induced Cell Oxidative Stress and Inflammatory Responses via Regulation of the TLR4-NF-κB Signaling Pathway in Bovine Rumen Epithelial Cells.Toxins (Basel). 2023 Aug 21;15(8):512. doi: 10.3390/toxins15080512. Toxins (Basel). 2023. PMID: 37624269 Free PMC article.
-
Anti-inflammatory activity of d-pinitol possibly through inhibiting COX-2 enzyme: in vivo and in silico studies.Front Chem. 2024 Apr 16;12:1366844. doi: 10.3389/fchem.2024.1366844. eCollection 2024. Front Chem. 2024. PMID: 38690012 Free PMC article.
-
Quercetin: A Natural Ally in Combating Breast Cancer.Int J Nanomedicine. 2025 Jul 19;20:9155-9177. doi: 10.2147/IJN.S518174. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40708864 Free PMC article. Review.
-
Biocompatibility and radiosensitivity of a fiber optical-based dosimeter: biological applications.Biomed Opt Express. 2024 Apr 30;15(5):3492-3506. doi: 10.1364/BOE.523849. eCollection 2024 May 1. Biomed Opt Express. 2024. PMID: 38855686 Free PMC article.
-
Selected Flavonols in Breast and Gynecological Cancer: A Systematic Review.Nutrients. 2023 Jun 28;15(13):2938. doi: 10.3390/nu15132938. Nutrients. 2023. PMID: 37447264 Free PMC article.
References
LinkOut - more resources
Full Text Sources

