Iron Promotes Cardiac Doxorubicin Retention and Toxicity Through Downregulation of the Mitochondrial Exporter ABCB8
- PMID: 35359834
- PMCID: PMC8963208
- DOI: 10.3389/fphar.2022.817951
Iron Promotes Cardiac Doxorubicin Retention and Toxicity Through Downregulation of the Mitochondrial Exporter ABCB8
Abstract
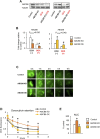
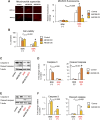
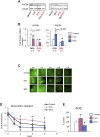
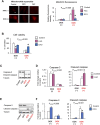
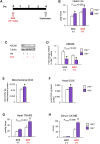
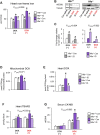
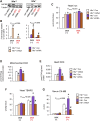
In several cancers, the efflux and resistance against doxorubicin (DOX), an effective anticancer drug, are associated with cellular iron deficiency and overexpression of the mitochondrial exporter ABCB8. Conversely, decreased ABCB8 expression and disrupted iron homeostasis in the heart have been implicated in DOX-associated cardiotoxicity. While studies have demonstrated that altered iron status can modulate the susceptibility to DOX cardiotoxicity, the exact molecular mechanisms have not been clearly understood. Here, we hypothesized that iron stores influence cardiac ABCB8 expression and consequently cardiac retention and toxicity of DOX. First, we found that ABCB8 deficiency in cardiomyocytes decreased DOX efflux, increased DOX-induced toxicity, and decreased cell viability. Conversely, intracellular DOX retention and toxicity were ameliorated by ABCB8 overexpression. To determine if altered cardiac iron status modifies ABCB8 expression, we treated cardiomyocytes with high iron or iron chelators. Western blot and qPCR analyses revealed that ABCB8 levels were decreased in iron overload and increased in iron deficiency. Subsequently, DOX retention and toxicity were increased in cardiomyocytes with iron overload, whereas iron deficiency ameliorated these effects. Next, we validated our results using a mouse model of hereditary hemochromatosis (HH), a genetic iron overload disorder. HH mice exhibited decreased ABCB8 expression and increased DOX retention and toxicity. These changes were abolished by the treatment of HH mice with a low-iron diet. Finally, cardiac-specific overexpression of ABCB8 in HH mice prevented cardiac DOX accumulation and abrogated DOX-induced cardiotoxicity without altering iron overload in the heart. Together, our results demonstrate that ABCB8 mediates DOX efflux and that iron regulates DOX retention and toxicity by altering cardiac ABCB8 expression. Our study identifies a novel role of iron in DOX-induced cardiotoxicity and suggests potential therapeutic intervention for DOX and anthracycline-based cancer pharmacology.
Keywords: cardiotoxicity; doxorubicin efflux; hemochromatosis; iron chelator; iron overload.
Copyright © 2022 Menon and Kim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Akimoto H., Bruno N. A., Slate D. L., Billingham M. E., Torti S. V., Torti F. M. (1993). Effect of Verapamil on Doxorubicin Cardiotoxicity: Altered Muscle Gene Expression in Cultured Neonatal Rat Cardiomyocytes. Cancer Res. 53 (19), 4658–4664. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

