Temporal changes in factors associated with COVID-19 vaccine hesitancy and uptake among adults in Hong Kong: Serial cross-sectional surveys
- PMID: 35359914
- PMCID: PMC8961079
- DOI: 10.1016/j.lanwpc.2022.100441
Temporal changes in factors associated with COVID-19 vaccine hesitancy and uptake among adults in Hong Kong: Serial cross-sectional surveys
Abstract
Background: COVID-19 vaccine hesitancy can lead to reduced vaccine uptake and hinder the safe relaxation of other public health measures. This study aims to explore the factors associated with vaccine hesitancy and uptake among adults before and after the implementation of the COVID-19 vaccination program in Hong Kong.
Methods: Cross-sectional telephone surveys were conducted every four weeks over a nine-month period from November 2020 through July 2021. Target respondents were Hong Kong resident aged 18 or above and recruited by random-digit dialling. In each survey, responses on COVID-19 vaccine hesitancy and COVID-19 vaccine uptake were collected as primary and secondary outcomes, respectively. Data of potentially associated factors, including socio-demographics, chronic medical conditions, perceived risk of COVID-19, perceived personal efficacy in self-protection, confidence in the government's ability to control the pandemic, compliance with social distancing measures, and confidence in COVID-19 vaccines, were also collected. Multivariable logistic regression models were used to examine the factors associated with COVID-19 vaccine hesitancy at different time points.
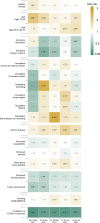
Findings: Ten cross-sectional surveys were conducted, including 7411 respondents. The levels of vaccine hesitancy fluctuated over time. From December 2020 to May 2021, the age group with the highest vaccine hesitancy was young adults 18-34y, while the vaccine hesitancy was highest among adults ≥ 65y in June-July 2021 (Fig. 2C). Our regression analyses (Fig. 3) showed that before and at the beginning of the rollout of the mass vaccination program, there was no statistically significant association between chronic medical conditions and vaccine hesitancy. However, two-five months after the program implementation respondents with chronic medical conditions were more likely to be hesitant. From January to June 2021, higher confidence in the government was associated with lower vaccine hesitancy (Fig. 3). Confidence in COVID-19 vaccines was consistently associated with lower vaccine hesitancy at different stages of the program.
Interpretation: The factors associated with COVID-19 vaccine hesitancy changed over time. This study highlighted the importance to monitor temporal changes in COVID-19 vaccine hesitancy and associated factors, and adjust promotion strategies correspondingly to boost vaccination uptake.
Funding: Health and Medical Research Fund, Hong Kong.
© 2022 The Author(s).
Conflict of interest statement
BJC consults for AstraZeneca, GSK, Moderna, Pfizer, Roche and Sanofi Pasteur. The authors report no other potential conflicts of interest.
Figures



References
LinkOut - more resources
Full Text Sources

