Notochordal Cell-Based Treatment Strategies and Their Potential in Intervertebral Disc Regeneration
- PMID: 35359916
- PMCID: PMC8963872
- DOI: 10.3389/fcell.2021.780749
Notochordal Cell-Based Treatment Strategies and Their Potential in Intervertebral Disc Regeneration
Abstract
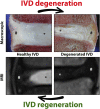
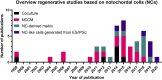
Chronic low back pain is the number one cause of years lived with disability. In about 40% of patients, chronic lower back pain is related to intervertebral disc (IVD) degeneration. The standard-of-care focuses on symptomatic relief, while surgery is the last resort. Emerging therapeutic strategies target the underlying cause of IVD degeneration and increasingly focus on the relatively overlooked notochordal cells (NCs). NCs are derived from the notochord and once the notochord regresses they remain in the core of the developing IVD, the nucleus pulposus. The large vacuolated NCs rapidly decline after birth and are replaced by the smaller nucleus pulposus cells with maturation, ageing, and degeneration. Here, we provide an update on the journey of NCs and discuss the cell markers and tools that can be used to study their fate and regenerative capacity. We review the therapeutic potential of NCs for the treatment of IVD-related lower back pain and outline important future directions in this area. Promising studies indicate that NCs and their secretome exerts regenerative effects, via increased proliferation, extracellular matrix production, and anti-inflammatory effects. Reports on NC-like cells derived from embryonic- or induced pluripotent-stem cells claim to have successfully generated NC-like cells but did not compare them with native NCs for phenotypic markers or in terms of their regenerative capacity. Altogether, this is an emerging and active field of research with exciting possibilities. NC-based studies demonstrate that cues from developmental biology can pave the path for future clinical therapies focused on regenerating the diseased IVD.
Keywords: Notochordal cell; cell therapeutic potential; conditioned media (CM); extracellular matrix (ECM); intervertebral disc – degeneration; low back pain; secretome.
Copyright © 2022 Bach, Poramba-Liyanage, Riemers, Guicheux, Camus, Iatridis, Chan, Ito, Le Maitre and Tryfonidou.
Conflict of interest statement
KI is a paid consultant and shareholder at NC Biomatrix BV. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Native nucleus pulposus tissue matrix promotes notochordal differentiation of human induced pluripotent stem cells with potential for treating intervertebral disc degeneration.J Biomed Mater Res A. 2015 Mar;103(3):1053-9. doi: 10.1002/jbm.a.35243. Epub 2014 Jun 17. J Biomed Mater Res A. 2015. PMID: 24889905
-
Human iPSCs can be differentiated into notochordal cells that reduce intervertebral disc degeneration in a porcine model.Theranostics. 2019 Oct 12;9(25):7506-7524. doi: 10.7150/thno.34898. eCollection 2019. Theranostics. 2019. PMID: 31695783 Free PMC article.
-
Notochordal cells: A potential therapeutic option for intervertebral disc degeneration.Cell Prolif. 2024 Feb;57(2):e13541. doi: 10.1111/cpr.13541. Epub 2023 Sep 11. Cell Prolif. 2024. PMID: 37697480 Free PMC article. Review.
-
Co-culturing nucleus pulposus mesenchymal stem cells with notochordal cell-rich nucleus pulposus explants attenuates tumor necrosis factor-α-induced senescence.Stem Cell Res Ther. 2018 Jun 26;9(1):171. doi: 10.1186/s13287-018-0919-9. Stem Cell Res Ther. 2018. PMID: 29941029 Free PMC article.
-
Differentiation of Pluripotent Stem Cells into Nucleus Pulposus Progenitor Cells for Intervertebral Disc Regeneration.Curr Stem Cell Res Ther. 2019;14(1):57-64. doi: 10.2174/1574888X13666180918095121. Curr Stem Cell Res Ther. 2019. PMID: 30227822 Review.
Cited by
-
Immunomorphogenesis in Degenerative Disc Disease: The Role of Proinflammatory Cytokines and Angiogenesis Factors.Biomedicines. 2023 Aug 3;11(8):2184. doi: 10.3390/biomedicines11082184. Biomedicines. 2023. PMID: 37626681 Free PMC article.
-
Isolation and tracing of matrix-producing notochordal and chondrocyte cells using ACAN-2A-mScarlet reporter human iPSC lines.Sci Adv. 2024 Oct 25;10(43):eadp3170. doi: 10.1126/sciadv.adp3170. Epub 2024 Oct 23. Sci Adv. 2024. PMID: 39441923 Free PMC article.
-
D-mannose alleviates intervertebral disc degeneration through glutamine metabolism.Mil Med Res. 2024 May 6;11(1):28. doi: 10.1186/s40779-024-00529-4. Mil Med Res. 2024. PMID: 38711073 Free PMC article.
-
A Combined Western and Bead-Based Multiplex Platform to Characterize Extracellular Vesicles.Tissue Eng Part C Methods. 2023 Nov;29(11):493-504. doi: 10.1089/ten.TEC.2023.0056. Epub 2023 Aug 22. Tissue Eng Part C Methods. 2023. PMID: 37470213 Free PMC article.
-
Alginate vs. Hyaluronic Acid as Carriers for Nucleus Pulposus Cells: A Study on Regenerative Outcomes in Disc Degeneration.Cells. 2024 Nov 30;13(23):1984. doi: 10.3390/cells13231984. Cells. 2024. PMID: 39682732 Free PMC article.
References
-
- Angerer P., Simon L., Tritschler S., Wolf F. A., Fischer D., Theis F. J. (2017). Single Cells Make Big Data: New Challenges and Opportunities in Transcriptomics. Curr. Opin. Syst. Biol. 4, 85–91. 10.1016/j.coisb.2017.07.004 - DOI
-
- Arkesteijn I. T., Smolders L. A., Spillekom S., Riemers F. M., Potier E., Meij B. P., et al. (2015). Effect of Coculturing Canine Notochordal, Nucleus Pulposus and Mesenchymal Stromal Cells for Intervertebral Disc Regeneration. Arthritis Res. Ther. 17 (1), 60–66. 10.1186/s13075-015-0569-6 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

