Natural SARS-CoV-2 Infection Affects Neutralizing Activity in Saliva of Vaccinees
- PMID: 35359971
- PMCID: PMC8962193
- DOI: 10.3389/fimmu.2022.820250
Natural SARS-CoV-2 Infection Affects Neutralizing Activity in Saliva of Vaccinees
Abstract
Background: SARS-CoV-2 transmission mainly occurs through exposure of the upper airway mucosa to infected secretions such as saliva, which are excreted by an infected person. Thus, oral mucosal immunity plays a central role in the prevention of and early defense against SARS-CoV-2 infection. Although virus-specific antibody response has been extensively investigated in blood samples of SARS-CoV-2-infected patients and vaccinees, local humoral immunity in the oral cavity and its relationship to systemic antibody levels needs to be further addressed.
Material and methods: We fine-tuned a virus neutralization assay (vNTA) to measure the neutralizing activity (NA) of plasma and saliva samples from 20 SARS-CoV-2-infected (SI), 40 SARS-CoV-2-vaccinated (SV), and 28 SARS-CoV-2-vaccinated subjects with a history of infection (SIV) using the "wild type" SARS-CoV-2 lineage B.1 (EU) and the Delta (B.1.617.2) strains. To validate the vNTA results, the presence of neutralizing antibodies (NAbs) to the spike receptor binding domain (RBD) was evaluated with an ELISA assay.
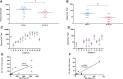
Results: NA to SARS-CoV-2 lineage B.1 (EU) was present in plasma samples from all the tested subjects, with higher titers in SIV compared to both SI and SV. Conversely, NA was detected in saliva samples from 10.3% SV, 45% SI, and 92.6% SIV, with significantly lower titers in SV compared to both SI and SIV. The detection of NAbs in saliva reflected its reduced NA in SV.
Discussion: The difference in NA of plasma vs. saliva was confirmed in a vNTA where the SARS-CoV-2 B.1 and Delta strains were tested head-to-head, which also revealed a reduced NA of both specimens compared to the B.1 variant.
Conclusions: The administration of SARS-CoV-2 vaccines was associated with limited virus NA in the oral cavity, as measured in saliva and in comparison to plasma. This difference was more evident in vaccinees without a history of SARS-CoV-2 infection, possibly highlighting the importance of local exposure at the site of virus acquisition to effectively prevent the infection and block its spread. Nevertheless, the presence of immune escape mutations as possibly represented by the SARS-CoV-2 Delta variant negatively affects both local and systemic efficacy of NA associated with vaccination.
Keywords: SARS-CoV-2; antibodies; neutralizing activity; saliva; variants.
Copyright © 2022 Garziano, Utyro, Poliseno, Santantonio, Saulle, Strizzi, Lo Caputo, Clerici, Introini and Biasin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Saliva and Plasma Neutralizing Activity Induced by the Administration of a Third bnt162b2 Vaccine Dose.Int J Mol Sci. 2022 Nov 18;23(22):14341. doi: 10.3390/ijms232214341. Int J Mol Sci. 2022. PMID: 36430815 Free PMC article.
-
Detection of Antibody Responses Against SARS-CoV-2 in Plasma and Saliva From Vaccinated and Infected Individuals.Front Immunol. 2021 Dec 20;12:759688. doi: 10.3389/fimmu.2021.759688. eCollection 2021. Front Immunol. 2021. PMID: 34987505 Free PMC article.
-
Antibody Avidity and Neutralizing Response against SARS-CoV-2 Omicron Variant after Infection or Vaccination.J Immunol Res. 2022 Aug 31;2022:4813199. doi: 10.1155/2022/4813199. eCollection 2022. J Immunol Res. 2022. PMID: 36093434 Free PMC article.
-
Neutralizing Antibodies Against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Variants Induced by Natural Infection or Vaccination: A Systematic Review and Pooled Analysis.Clin Infect Dis. 2022 Mar 1;74(4):734-742. doi: 10.1093/cid/ciab646. Clin Infect Dis. 2022. PMID: 34302458 Free PMC article.
-
The "oral" history of COVID-19: Primary infection, salivary transmission, and post-acute implications.J Periodontol. 2021 Oct;92(10):1357-1367. doi: 10.1002/JPER.21-0277. Epub 2021 Sep 7. J Periodontol. 2021. PMID: 34390597 Free PMC article. Review.
Cited by
-
Differentially induced immunity in buccal and nasal mucosae after vaccination for SARS-CoV-2: Prospects for mass scale immunity-screening in large populations.Front Immunol. 2022 Nov 17;13:999693. doi: 10.3389/fimmu.2022.999693. eCollection 2022. Front Immunol. 2022. PMID: 36466833 Free PMC article.
-
Humoral and cell-mediated immune responses in HIV-vertically infected young patients after three doses of the BNT162b2 mRNA SARS-CoV-2 vaccine.Front Immunol. 2024 Jan 4;14:1301766. doi: 10.3389/fimmu.2023.1301766. eCollection 2023. Front Immunol. 2024. PMID: 38250079 Free PMC article.
-
Updated Considerations for the Immunopharmacological Aspects of the "Talented mRNA Vaccines".Vaccines (Basel). 2023 Sep 12;11(9):1481. doi: 10.3390/vaccines11091481. Vaccines (Basel). 2023. PMID: 37766157 Free PMC article. Review.
-
Antiviral effect of SARS-CoV-2 N-specific CD8+ T cells induced in lungs by engineered extracellular vesicles.NPJ Vaccines. 2023 Jun 2;8(1):83. doi: 10.1038/s41541-023-00686-y. NPJ Vaccines. 2023. PMID: 37268624 Free PMC article.
-
Saliva and Plasma Neutralizing Activity Induced by the Administration of a Third bnt162b2 Vaccine Dose.Int J Mol Sci. 2022 Nov 18;23(22):14341. doi: 10.3390/ijms232214341. Int J Mol Sci. 2022. PMID: 36430815 Free PMC article.
References
-
- A Mucosal Antibody Response is Induced by Intra-Muscular SARS-CoV-2 mRNA Vaccination, in: Medrxiv. Available at: https://www.medrxiv.org/content/10.1101/2021.08.01.21261297v2 (Accessed November 19, 2021).
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

