Re-Endothelialization of Decellularized Liver Scaffolds: A Step for Bioengineered Liver Transplantation
- PMID: 35360393
- PMCID: PMC8960611
- DOI: 10.3389/fbioe.2022.833163
Re-Endothelialization of Decellularized Liver Scaffolds: A Step for Bioengineered Liver Transplantation
Abstract
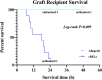
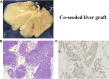
Bioengineered livers (BELs) are an attractive therapeutic alternative to address the donor organ shortage for liver transplantation. The goal of BELs technology aims at replacement or regeneration of the native human liver. A variety of approaches have been proposed for tissue engineering of transplantable livers; the current review will highlight the decellularization-recellularization approach to BELs. For example, vascular patency and appropriate cell distribution and expansion are critical components in the production of successful BELs. Proper solutions to these components of BELs have challenged its development. Several strategies, such as heparin immobilization, heparin-gelatin, REDV peptide, and anti-CD31 aptamer have been developed to extend the vascular patency of revascularized bioengineered livers (rBELs). Other novel methods have been developed to enhance cell seeding of parenchymal cells and to increase graft functionality during both bench and in vivo perfusion. These enhanced methods have been associated with up to 15 days of survival in large animal (porcine) models of heterotopic transplantation but have not yet permitted extended survival after implantation of BELs in the orthotopic position. This review will highlight both the remaining challenges and the potential for clinical application of functional bioengineered grafts.
Keywords: bioengineered livers (BELs); decellularization; heterotopic transplantation; liver transplantation; orthotopic transplantation; scaffolds.
Copyright © 2022 Li, Tharwat, Larson, Felgendreff, Hosseiniasl, Rmilah, Safwat, Ross and Nyberg.
Conflict of interest statement
JR was employed by Miromatrix Medical Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer JB declared a shared affiliation, with no collaboration, with one of the authors KL to the handling editor at the time of the review.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

