Nanomaterial-Based Immunocapture Platforms for the Recognition, Isolation, and Detection of Circulating Tumor Cells
- PMID: 35360401
- PMCID: PMC8964261
- DOI: 10.3389/fbioe.2022.850241
Nanomaterial-Based Immunocapture Platforms for the Recognition, Isolation, and Detection of Circulating Tumor Cells
Abstract
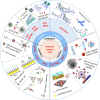
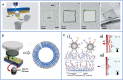
Circulating tumor cells (CTCs) are a type of cancer cells that circulate in the peripheral blood after breaking away from solid tumors and are essential for the establishment of distant metastasis. Up to 90% of cancer-related deaths are caused by metastatic cancer. As a new type of liquid biopsy, detecting and analyzing CTCs will provide insightful information for cancer diagnosis, especially the in-time disease status, which would avoid some flaws and limitations of invasive tissue biopsy. However, due to the extremely low levels of CTCs among a large number of hematologic cells, choosing immunocapture platforms for CTC detection and isolation will achieve good performance with high purity, selectivity, and viability. These properties are directly associated with precise downstream analysis of CTC profiling. Recently, inspired by the nanoscale interactions of cells in the tissue microenvironment, platforms based on nanomaterials have been widely explored to efficiently enrich and sensitively detect CTCs. In this review, various immunocapture platforms based on different nanomaterials for efficient isolation and sensitive detection of CTCs are outlined and discussed. First, the design principles of immunoaffinity nanomaterials are introduced in detail. Second, the immunocapture and release of platforms based on nanomaterials ranging from nanoparticles, nanostructured substrates, and immunoaffinity microfluidic chips are summarized. Third, recent advances in single-cell release and analysis of CTCs are introduced. Finally, some perspectives and challenges are provided in future trends of CTC studies.
Keywords: biological detection; circulating tumor cells; immunocapture platform; liquid biopsy; nanomaterials.
Copyright © 2022 Liu, Li, Zhang and Guo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures









References
-
- Ashworth T. (1869). A Case of Cancer in Which Cells Similar to Those in the Tumours Were Seen in the Blood after Death. Aust. Med. J. 14, 146.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

