R3-AFP score is a new composite tool to refine prediction of hepatocellular carcinoma recurrence after liver transplantation
- PMID: 35360522
- PMCID: PMC8961219
- DOI: 10.1016/j.jhepr.2022.100445
R3-AFP score is a new composite tool to refine prediction of hepatocellular carcinoma recurrence after liver transplantation
Abstract
Background & aims: Patients with hepatocellular carcinoma (HCC) are selected for liver transplantation (LT) based on pre-LT imaging ± alpha-foetoprotein (AFP) level, but discrepancies between pre-LT tumour assessment and explant are frequent. Our aim was to design an explant-based recurrence risk reassessment score to refine prediction of recurrence after LT and provide a framework to guide post-LT management.
Methods: Adult patients who underwent transplantation between 2000 and 2018 for HCC in 47 centres were included. A prediction model for recurrence was developed using competing-risk regression analysis in a European training cohort (TC; n = 1,359) and tested in a Latin American validation cohort (VC; n=1,085).
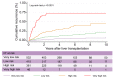
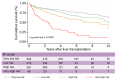
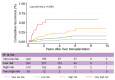
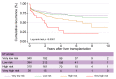
Results: In the TC, 76.4% of patients with HCC met the Milan criteria, and 89.9% had an AFP score of ≤2 points. The recurrence risk reassessment (R3)-AFP model was designed based on variables independently associated with recurrence in the TC (with associated weights): ≥4 nodules (sub-distribution of hazard ratio [SHR] = 1.88, 1 point), size of largest nodule (3-6 cm: SHR = 1.83, 1 point; >6 cm: SHR = 5.82, 5 points), presence of microvascular invasion (MVI; SHR = 2.69, 2 points), nuclear grade >II (SHR = 1.20, 1 point), and last pre-LT AFP value (101-1,000 ng/ml: SHR = 1.57, 1 point; >1,000 ng/ml: SHR = 2.83, 2 points). Wolber's c-index was 0.76 (95% CI 0.72-0.80), significantly superior to an R3 model without AFP (0.75; 95% CI 0.72-0.79; p = 0.01). Four 5-year recurrence risk categories were identified: very low (score = 0; 5.5%), low (1-2 points; 15.1%), high (3-6 points; 39.1%), and very high (>6 points; 73.9%). The R3-AFP score performed well in the VC (Wolber's c-index of 0.78; 95% CI 0.73-0.83).
Conclusions: The R3 score including the last pre-LT AFP value (R3-AFP score) provides a user-friendly, standardised framework to design post-LT surveillance strategies, protocols, or adjuvant therapy trials for HCC not limited to the Milan criteria.
Clinical trials registration: NCT03775863.
Lay summary: Considering discrepancies between pre-LT tumour assessment and explant are frequent, reassessing the risk of recurrence after LT is critical to further refine the management of patients with HCC. In a large and international cohort of patients who underwent transplantation for HCC, we designed and validated the R3-AFP model based on variables independently associated with recurrence post-LT (number of nodules, size of largest nodule, presence of MVI, nuclear grade, and last pre-LT AFP value). The R3-AFP model including last available pre-LT AFP value outperformed the original R3 model only based on explant features. The final R3-AFP scoring system provides a robust framework to design post-LT surveillance strategies, protocols, or adjuvant therapy trials, irrespective of criteria used to select patients with HCC for LT.
Keywords: AFP, alpha-foetoprotein; Explants pathology; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; LT, liver transplantation; Liver cancer; Liver transplantation; MVI, microvascular invasion; Prediction; R3, recurrence risk reassessment; RETREAT, Risk Estimation of Tumour Recurrence After Transplant; Recurrence; SHR, sub-distribution of hazard ratio; TC, test cohort; TTR, time to recurrence; VC, validation cohort.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest that pertain to this work. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures

References
-
- Llovet J.M., Kelley R.K., Villanueva A., Singal A.G., Pikarsky E., Roayaie S., et al. Hepatocellular carcinoma. Nat Rev Dis Prim. 2021;7:6. - PubMed
-
- Llovet J.M., Fuster J., Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434–1440. - PubMed
-
- Mazzaferro V., Regalia E., Doci R., Andreola S., Pulvirenti A., Bozzetti F., et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. - PubMed
-
- Mazzaferro V., Llovet J.M., Miceli R., Bhoori S., Schiavo M., Mariani L., et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

