A COVID-19 vaccine candidate composed of the SARS-CoV-2 RBD dimer and Neisseria meningitidis outer membrane vesicles
- PMID: 35360883
- PMCID: PMC8826971
- DOI: 10.1039/d1cb00200g
A COVID-19 vaccine candidate composed of the SARS-CoV-2 RBD dimer and Neisseria meningitidis outer membrane vesicles
Abstract
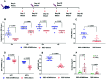
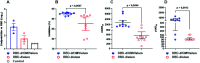
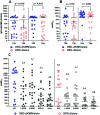
SARS-CoV-2 infection is mediated by the interaction of the spike glycoprotein trimer via its receptor-binding domain (RBD) with the host's cellular receptor. Vaccines seek to block this interaction by eliciting neutralizing antibodies, most of which are directed toward the RBD. Many protein subunit vaccines require powerful adjuvants to generate a potent antibody response. Here, we report on the use of a SARS-CoV-2 dimeric recombinant RBD combined with Neisseria meningitidis outer membrane vesicles (OMVs), adsorbed on alum, as a promising COVID-19 vaccine candidate. This formulation induces a potent and neutralizing immune response in laboratory animals, which is higher than that of the dimeric RBD alone adsorbed on alum. Sera of people vaccinated with this vaccine candidate, named Soberana01, show a high inhibition level of the RBD-ACE2 interaction using RBD mutants corresponding to SARS-CoV-2 variants of concern and wild-type expressed using the phage display technology. To our knowledge, this is the first time that the immunostimulation effect of N. meningitidis OMVs is evaluated in vaccine candidates against SARS-CoV-2.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no financial conflicts of interest. Some of the authors are co-inventors on a provisional SARS-CoV-2 vaccine patent (Cu 2020-57) based on these results.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

