One- and Two-Photon Activated Release of Oxaliplatin from a Pt(IV)-Functionalized Poly(phenylene ethynylene)
- PMID: 35360898
- PMCID: PMC12124872
- DOI: 10.1021/acsami.2c00859
One- and Two-Photon Activated Release of Oxaliplatin from a Pt(IV)-Functionalized Poly(phenylene ethynylene)
Abstract
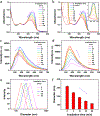
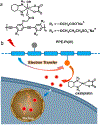
We report a water-soluble poly(phenylene ethynylene) (PPE-Pt(IV)) that is functionalized with oxidized oxaliplatin Pt(IV) units and its use for photoactivated chemotherapy. The photoactivation strategy is based on photoinduced electron transfer from the PPE backbone to oxaliplatin Pt(IV) as an electron acceptor; this process triggers the release of oxaliplatin, which is a clinically used anticancer drug. Mechanistic studies carried out using steady-state and time-resolved fluorescence spectroscopy coupled with picosecond-nanosecond transient absorption support the hypothesis that electron transfer triggers the drug release. Photoactivation is effective, producing oxaliplatin with a good chemical yield in less than 1 h of photolysis (400 nm, 5 mW cm-2). Photorelease of oxaliplatin from PPE-Pt(IV) can also be effected with two-photon excitation by using 100 fs pulsed light at 725 nm. Cytotoxicity studies using SK-OV-3 human ovarian cancer cells demonstrate that without photoactivation PPE-Pt(IV) is not cytotoxic at concentrations up to 10 μM in polymer repeating unit (PRU) concentration. However, following a short period of 460 nm irradiation, oxaliplatin is released from PPE-Pt(IV), resulting in cytotoxicity at concentrations as low as 2.5 μM PRU.
Keywords: oxaliplatin; photoactivated chemotherapy; photoinduced electron transfer; two-photon absorption; water-soluble poly(phenylene ethynylene).
Conflict of interest statement
Notes
The authors declare no competing financial interest.
Figures



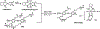

References
-
- Huang H; Delikanli S; Zeng H; Ferkey DM; Pralle A Remote Control of Ion Channels and Neurons through Magnetic-Field Heating of Nanoparticles. Nat. Nanotechnol 2010, 5 (8), 602–606. - PubMed
-
- Krawczyk K; Xue S; Buchmann P; Charpin-El-Hamri G; Saxena P; Hussherr M-D; Shao J; Ye H; Xie M; Fussenegger M Electrogenetic Cellular Insulin Release for Real-Time Glycemic Control in Type 1 Diabetic Mice. Science 2020, 368 (6494), 993–1001. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

