Headache onset after vaccination against SARS-CoV-2: a systematic literature review and meta-analysis
- PMID: 35361131
- PMCID: PMC8969402
- DOI: 10.1186/s10194-022-01400-4
Headache onset after vaccination against SARS-CoV-2: a systematic literature review and meta-analysis
Abstract
Background: Vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are used to reduce the risk of developing Coronavirus Disease 2019 (COVID-19). Despite the significant benefits in terms of reduced risk of hospitalization and death, different adverse events may present after vaccination: among them, headache is one of the most common, but nowadays there is no summary presentation of its incidence and no description of its main features.
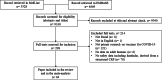
Methods: We searched PubMed and EMBASE covering the period between January 1st 2020 and August 6th, 2021, looking for record in English and with an abstract and using three main search terms (with specific variations): COVID-19/SARS-CoV-2; Vaccination; headache/adverse events. We selected manuscript including information on subjects developing headache after injection, and such information had to be derived from a structured form (i.e. no free reporting). Pooled estimates and 95% confidence intervals were calculated. Analyses were carried out by vaccine vs. placebo, by first vs. second dose, and by mRNA-based vs. "traditional" vaccines; finally, we addressed the impact of age and gender on post-vaccine headache onset.
Results: Out of 9338 records, 84 papers were included in the review, accounting for 1.57 million participants, 94% of whom received BNT162b2 or ChAdOx1. Headache was generally the third most common AE: it was detected in 22% (95% CI 18-27%) of subjects after the first dose of vaccine and in 29% (95% CI 23-35%) after the second, with an extreme heterogeneity. Those receiving placebo reported headache in 10-12% of cases. No differences were detected across different vaccines or by mRNA-based vs. "traditional" ones. None of the studies reported information on headache features. A lower prevalence of headache after the first injection of BNT162b2 among older participants was shown.
Conclusions: Our results show that vaccines are associated to a two-fold risk of developing headache within 7 days from injection, and the lack of difference between vaccine types enable to hypothesize that headache is secondary to systemic immunological reaction than to a vaccine-type specific reaction. Some descriptions report onset within the first 24 h and that in around one-third of the cases, headache has migraine-like features with pulsating quality, phono and photophobia; in 40-60% of the cases aggravation with activity is observed. The majority of patients used some medication to treat headache, the one perceived as the most effective being acetylsalicylic acid.
Keywords: Adverse Event; BNT162b2; COVID-19; ChAdOx1; Headache; SARS-CoV-2; Vaccination.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Similar articles
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
Efficacy and safety of COVID-19 vaccines.Cochrane Database Syst Rev. 2022 Dec 7;12(12):CD015477. doi: 10.1002/14651858.CD015477. Cochrane Database Syst Rev. 2022. PMID: 36473651 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Risk of myocarditis and pericarditis after a COVID-19 mRNA vaccine booster and after COVID-19 in those with and without prior SARS-CoV-2 infection: A self-controlled case series analysis in England.PLoS Med. 2023 Jun 7;20(6):e1004245. doi: 10.1371/journal.pmed.1004245. eCollection 2023 Jun. PLoS Med. 2023. PMID: 37285378 Free PMC article.
-
SARS-CoV-2-neutralising monoclonal antibodies to prevent COVID-19.Cochrane Database Syst Rev. 2022 Jun 17;6(6):CD014945. doi: 10.1002/14651858.CD014945.pub2. Cochrane Database Syst Rev. 2022. PMID: 35713300 Free PMC article.
Cited by
-
Evaluation of Short-Term Symptoms Associated With COVID-19 Vaccines Used Among Adolescents in Saudi Arabia.Cureus. 2022 Sep 18;14(9):e29306. doi: 10.7759/cureus.29306. eCollection 2022 Sep. Cureus. 2022. PMID: 36277554 Free PMC article.
-
Adverse Events of COVID-19 Vaccines in the United States: Temporal and Spatial Analysis.JMIR Public Health Surveill. 2024 Jul 15;10:e51007. doi: 10.2196/51007. JMIR Public Health Surveill. 2024. PMID: 39008362 Free PMC article.
-
Cost-efficacy evaluation of a mindfulness-based program added to treatment as usual for the treatment of chronic migraine associated to medication overuse headache.Neurol Sci. 2025 Jun 25. doi: 10.1007/s10072-025-08327-z. Online ahead of print. Neurol Sci. 2025. PMID: 40555938 No abstract available.
-
COVID-19 Vaccines Adverse Reactions Reported to the Pharmacovigilance Unit of Beira Interior in Portugal.J Clin Med. 2022 Sep 23;11(19):5591. doi: 10.3390/jcm11195591. J Clin Med. 2022. PMID: 36233459 Free PMC article.
-
Group of longitudinal adverse event patterns after the fourth dose of COVID-19 vaccination with a latent class analysis.Front Public Health. 2024 Jul 30;12:1406315. doi: 10.3389/fpubh.2024.1406315. eCollection 2024. Front Public Health. 2024. PMID: 39139673 Free PMC article.
References
-
- WHO coronavirus disease (COVID-19) dashboard. https://covid19.who.int/table
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous