Irinotecan plus temozolomide in relapsed Ewing sarcoma: an integrated analysis of retrospective studies
- PMID: 35361149
- PMCID: PMC8969362
- DOI: 10.1186/s12885-022-09469-5
Irinotecan plus temozolomide in relapsed Ewing sarcoma: an integrated analysis of retrospective studies
Abstract
Background: The prognosis of patients with relapsed Ewing sarcoma is poor. In this study, we aimed to pooled-analyze the efficacy and safety of the combination of irinotecan and temozolomide in treating patients with relapsed Ewing sarcoma.
Methods: PubMed, Cochrane CENTRAL, Web of Science, and EMBASE were systematically searched on September 27, 2021. The primary outcomes were rates of objective response and disease control, and the secondary outcomes were toxicities.
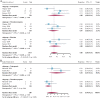
Results: Six retrospective studies with 184 patients were enrolled in the analysis. The median age ranged from 14 to 21. The integrated rates were 44% (95% confidence interval [CI] 31-58) for objective response and 66% (55-77) for disease control. Grade 3-4 neutropenia, thrombocytopenia, and diarrhea occurred in 8% (3-16), 7% (3-11), and 8% (5-10) of chemotherapeutic cycles, respectively. 18% (7-32) and 6% (2-11) of patients suffered grade 3-4 neutropenia and thrombocytopenia after irinotecan plus temozolomide treatment.
Conclusion: Irinotecan plus temozolomide combination chemotherapy showed antitumor activity and an acceptable safety profile in patients with relapsed Ewing sarcoma. More future prospective studies are needed to confirm the retrospective results.
Keywords: Chemotherapy; Ewing sarcoma; Integrated analysis; Irinotecan; Temozolomide.
© 2022. The Author(s).
Conflict of interest statement
No conflict of interest.
Figures


References
-
- Nesbit ME Jr, Gehan EA, Burgert EO Jr, Vietti TJ, Cangir A, Tefft M, Evans R, Thomas P, Askin FB, Kissane JM, et al. Multimodal therapy for the management of primary, nonmetastatic Ewing’s sarcoma of bone: a long-term follow-up of the first Intergroup study. J Clin Oncol. 1990;8(10):1664–74. - PubMed
-
- Grier HE, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, Gebhardt MC, Dickman PS, Perlman EJ, Meyers PA, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348(8):694–701. - PubMed
-
- Womer RB, West DC, Krailo MD, Dickman PS, Pawel BR, Grier HE, Marcus K, Sailer S, Healey JH, Dormans JP, et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the children’s oncology group. J Clin Oncol. 2012;30(33):4148–54. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

