Protocol for SYNchronising Exercises, Remedies in GaIt and Cognition at Home (SYNERGIC@Home): feasibility of a home-based double-blind randomised controlled trial to improve gait and cognition in individuals at risk for dementia
- PMID: 35361653
- PMCID: PMC8971768
- DOI: 10.1136/bmjopen-2021-059988
Protocol for SYNchronising Exercises, Remedies in GaIt and Cognition at Home (SYNERGIC@Home): feasibility of a home-based double-blind randomised controlled trial to improve gait and cognition in individuals at risk for dementia
Abstract
Introduction: Physical exercise and cognitive training have the potential to enhance cognitive function and mobility in older adults at risk of Alzheimer's disease and related dementia (ADRD), but little is known about the feasibility of delivering multidomain interventions in home settings of older adults at risk of ADRD. This study aims to assess the feasibility of home-based delivery of exercise and cognitive interventions, and to evaluate the relationship between participants' intervention preferences and their subsequent adherence. Secondary objectives include the effect of the interventions on ADRD risk factors, including frailty, mobility, sleep, diet and psychological health.
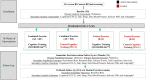
Methods and analysis: The SYNchronising Exercises, Remedies in GaIt and Cognition at Home (SYNERGIC@Home) feasibility trial is a randomised control trial that follows a 2×2 factorial design, with a 16-week home-based intervention programme (3 sessions per week) of physical exercises and cognitive training. Participants will be randomised in blocks of four to one of the following four arms: (1) combined exercise (aerobic and resistance)+cognitive training (NEUROPEAK); (2) combined exercise+control cognitive training (web searching); (3) control exercise (balance and toning)+cognitive training; and (4) control exercise+control cognitive training. SYNERGIC@Home will be implemented through video conferencing. Baseline and post-intervention assessments at 4-month and 10-month follow-up will include measures of cognition, frailty, mobility, sleep, diet and psychological health. Primary feasibility outcome is adherence to the interventions. Primary analytic outcome is the relationship between pre-allocation preference for a given intervention and subsequent adherence to the allocated intervention. A series of secondary analytic outcomes examining the potential effect of the individual and combined interventions on cognitive, mobility and general well-being will be measured at baseline and follow-up.
Ethics and dissemination: Ethics approval was granted by the relevant research ethics boards. Findings of the study will be presented to stakeholders and published in peer-reviewed journals and at provincial, national and international conferences.
Trial registration number: NCT04997681, Pre-results.
Keywords: Dementia; GERIATRIC MEDICINE; Neuropathology; Physiology.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
-
- Alzheimer Disease International . The Global Impact of Dementia: An analysis of prevalence, incidence, cost and trends. In: The world Alzheimer report, 2016.
-
- Albert MS, DeKosky ST, Dickson D, et al. . The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:270–9. 10.1016/j.jalz.2011.03.008 - DOI - PMC - PubMed
