Roxadustat Versus Epoetin Alfa for Treating Anemia in Patients with Chronic Kidney Disease on Dialysis: Results from the Randomized Phase 3 ROCKIES Study
- PMID: 35361724
- PMCID: PMC8970450
- DOI: 10.1681/ASN.2020111638
Roxadustat Versus Epoetin Alfa for Treating Anemia in Patients with Chronic Kidney Disease on Dialysis: Results from the Randomized Phase 3 ROCKIES Study
Abstract
Background: Concerns regarding cardiovascular safety with current treatments for anemia in patients with dialysis-dependent (DD)-CKD have encouraged the development of alternatives. Roxadustat, an oral hypoxia-inducible factor prolyl hydroxylase inhibitor, stimulates erythropoiesis by increasing endogenous erythropoietin and iron availability.
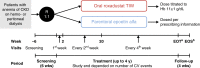
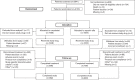
Methods: In this open-label phase 3 study, patients with DD-CKD and anemia were randomized 1:1 to oral roxadustat three times weekly or parenteral epoetin alfa per local clinic practice. Initial roxadustat dose depended on erythropoiesis-stimulating agent dose at screening for patients already on them and was weight-based for those not on them. The primary efficacy end point was mean hemoglobin change from baseline averaged over weeks 28‒52 for roxadustat versus epoetin alfa, regardless of rescue therapy use, tested for noninferiority (margin, -0.75 g/dl). Adverse events (AEs) were assessed.
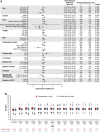
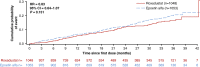
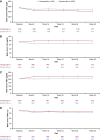
Results: Among 2133 patients randomized (n=1068 roxadustat, n=1065 epoetin alfa), mean age was 54.0 years, and 89.1% and 10.8% were on hemodialysis and peritoneal dialysis, respectively. Mean (95% confidence interval) hemoglobin change from baseline was 0.77 (0.69 to 0.85) g/dl with roxadustat and 0.68 (0.60 to 0.76) g/dl with epoetin alfa, demonstrating noninferiority (least squares mean difference [95% CI], 0.09 [0.01 to 0.18]; P<0.001). The proportion of patients experiencing ≥1 AE and ≥1 serious AE was 85.0% and 57.6% with roxadustat and 84.5% and 57.5% with epoetin alfa, respectively.
Conclusions: Roxadustat effectively increased hemoglobin in patients with DD-CKD, with an AE profile comparable to epoetin alfa.
Clinical trial registry name and registration number: Safety and Efficacy Study of Roxadustat to Treat Anemia in Patients With Chronic Kidney Disease, on Dialysis.
Clinicaltrials: gov Identifier: NCT02174731.
Keywords: HIF-PH inhibitor; anemia; chronic kidney disease; dialysis; roxadustat.
Copyright © 2022 by the American Society of Nephrology.
Figures

Comment in
-
The Search for the Perfect Agent for Anemia Management in Chronic Kidney Disease.J Am Soc Nephrol. 2022 Apr;33(4):662-664. doi: 10.1681/ASN.2022020173. J Am Soc Nephrol. 2022. PMID: 35361722 Free PMC article. No abstract available.
References
-
- Shih HM, Wu CJ, Lin SL: Physiology and pathophysiology of renal erythropoietin-producing cells. J Formos Med Assoc 117: 955–963, 2018 - PubMed
-
- Gafter-Gvili A, Schechter A, Rozen-Zvi B: Iron deficiency anemia in chronic kidney disease. Acta Haematol 142: 44–50, 2019 - PubMed
-
- Thorp ML, Johnson ES, Yang X, Petrik AF, Platt R, Smith DH: Effect of anaemia on mortality, cardiovascular hospitalizations and end-stage renal disease among patients with chronic kidney disease. Nephrology (Carlton) 14: 240–246, 2009 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

