High resolution optical mapping of cardiac electrophysiology in pre-clinical models
- PMID: 35361792
- PMCID: PMC8971487
- DOI: 10.1038/s41597-022-01253-1
High resolution optical mapping of cardiac electrophysiology in pre-clinical models
Erratum in
-
Publisher Correction: High resolution optical mapping of cardiac electrophysiology in pre-clinical models.Sci Data. 2024 Jan 18;11(1):93. doi: 10.1038/s41597-024-02941-w. Sci Data. 2024. PMID: 38238379 Free PMC article. No abstract available.
Abstract
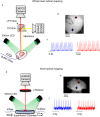
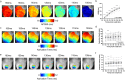
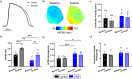
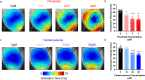
Optical mapping of animal models is a widely used technique in pre-clinical cardiac research. It has several advantages over other methods, including higher spatial resolution, contactless recording and direct visualisation of action potentials and calcium transients. Optical mapping enables simultaneous study of action potential and calcium transient morphology, conduction dynamics, regional heterogeneity, restitution and arrhythmogenesis. In this dataset, we have optically mapped Langendorff perfused isolated whole hearts (mouse and guinea pig) and superfused isolated atria (mouse). Raw datasets (consisting of over 400 files) can be combined with open-source software for processing and analysis. We have generated a comprehensive post-processed dataset characterising the baseline cardiac electrophysiology in these widely used pre-clinical models. This dataset also provides reference information detailing the effect of heart rate, clinically used anti-arrhythmic drugs, ischaemia-reperfusion and sympathetic nervous stimulation on cardiac electrophysiology. The effects of these interventions can be studied in a global or regional manner, enabling new insights into the prevention and initiation of arrhythmia.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

