Double helical π-aggregate nanoarchitectonics for amplified circularly polarized luminescence
- PMID: 35361805
- PMCID: PMC8971395
- DOI: 10.1038/s41467-022-29396-0
Double helical π-aggregate nanoarchitectonics for amplified circularly polarized luminescence
Abstract
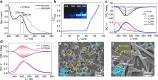
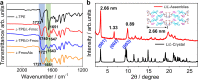
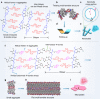
The canonical double helical π-stacked array of base pairs within DNA interior has inspired the interest in supramolecular double helical architectures with advanced electronic, magnetic and optical functions. Here, we report a selective-recognized and chirality-matched co-assembly strategy for the fabrication of fluorescent π-amino acids into double helical π-aggregates, which show exceptional strong circularly polarized luminescence (CPL). The single crystal structure of the optimal combination of co-assemblies shows that the double-stranded helical organization of these π-amino acids is cooperatively assisted by both CH-π and hydrogen-bond arrays with chirality match. The well-defined spatial arrangement of the π-chromophores could effectively suppress the non-radiative decay pathways and facilitate chiral exciton couplings, leading to superior CPL with a strong figure of merit (glum = 0.14 and QY = 0.76). Our findings might open a new door for developing DNA-inspired chiroptical materials with prominent properties by enantioselective co-assembly initiated double helical π-aggregation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Self-Assembly of Peptide Hierarchical Helical Arrays with Sequence-Encoded Circularly Polarized Luminescence.Nano Lett. 2021 Aug 11;21(15):6406-6415. doi: 10.1021/acs.nanolett.1c00697. Epub 2021 May 20. Nano Lett. 2021. PMID: 34014681
-
New Perspectives to Trigger and Modulate Circularly Polarized Luminescence of Complex and Aggregated Systems: Energy Transfer, Photon Upconversion, Charge Transfer, and Organic Radical.Acc Chem Res. 2020 Jul 21;53(7):1279-1292. doi: 10.1021/acs.accounts.0c00112. Epub 2020 Jul 10. Acc Chem Res. 2020. PMID: 32649172
-
Helical Nanostructures with Circularly Polarized Luminescence from the Multicomponent Assembly of π-Conjugated N-terminal Amino Acids.Chempluschem. 2020 Jul;85(7):1511-1522. doi: 10.1002/cplu.202000397. Chempluschem. 2020. PMID: 32644303 Review.
-
Fluorescent Dye-Based Chiral Crystalline Organic Salt Networks for Circularly Polarized Luminescence.Small. 2025 Jan;21(1):e2408874. doi: 10.1002/smll.202408874. Epub 2024 Oct 24. Small. 2025. PMID: 39449222
-
Helically assembled π-conjugated polymers with circularly polarized luminescence.Sci Technol Adv Mater. 2014 Aug 21;15(4):044203. doi: 10.1088/1468-6996/15/4/044203. eCollection 2014 Aug. Sci Technol Adv Mater. 2014. PMID: 27877698 Free PMC article. Review.
Cited by
-
Modulation of supramolecular chirality by stepwise axial coordination in a nano-size trizinc(ii)porphyrin trimer.Chem Sci. 2023 May 10;14(22):6032-6038. doi: 10.1039/d3sc00858d. eCollection 2023 Jun 7. Chem Sci. 2023. PMID: 37293642 Free PMC article.
-
Supramolecular rosette intermediated homochiral double helix.Nat Commun. 2025 Feb 17;16(1):1698. doi: 10.1038/s41467-025-57059-3. Nat Commun. 2025. PMID: 39962065 Free PMC article.
-
Enantio- and Diastereoselective Synthesis and Spiral-Stair-Like Single Helix Assembly of Figure-Eight Cyclophenylenes.Angew Chem Int Ed Engl. 2025 May 26;64(22):e202502764. doi: 10.1002/anie.202502764. Epub 2025 Apr 7. Angew Chem Int Ed Engl. 2025. PMID: 40104859 Free PMC article.
-
Cooperative supramolecular polymerization of styrylpyrenes for color-dependent circularly polarized luminescence and photocycloaddition.Nat Commun. 2023 Dec 4;14(1):8022. doi: 10.1038/s41467-023-43830-x. Nat Commun. 2023. PMID: 38049414 Free PMC article.
-
Bis-Pyridine-Based Organogel with AIE Effect and Sensing Performance towards Hg2.Gels. 2022 Jul 25;8(8):464. doi: 10.3390/gels8080464. Gels. 2022. PMID: 35892723 Free PMC article.
References
-
- Liljas, A. et al. Textbook of Structural Biology (World Scientific Publishing, 2009).
-
- Zhao D, van Leeuwen T, Cheng J, Feringa BL. Dynamic control of chirality and self-assembly of double-stranded helicates with light. Nat. Chem. 2017;9:250–256. - PubMed
-
- Huang Z, et al. Pulsating tubules from noncovalent macrocycles. Science. 2012;337:1521–1526. - PubMed
-
- Jones CD, et al. Braiding, branching and chiral amplification of nanofibres in supramolecular gels. Nat. Chem. 2019;11:375–381. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

