SARS-CoV-2 spike protein induces cognitive deficit and anxiety-like behavior in mouse via non-cell autonomous hippocampal neuronal death
- PMID: 35361832
- PMCID: PMC8970073
- DOI: 10.1038/s41598-022-09410-7
SARS-CoV-2 spike protein induces cognitive deficit and anxiety-like behavior in mouse via non-cell autonomous hippocampal neuronal death
Abstract
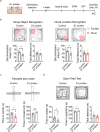
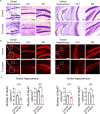
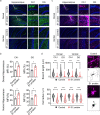
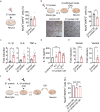
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is accompanied by chronic neurological sequelae such as cognitive decline and mood disorder, but the underlying mechanisms have not yet been elucidated. We explored the possibility that the brain-infiltrating SARS-CoV-2 spike protein contributes to the development of neurological symptoms observed in COVID-19 patients in this study. Our behavioral study showed that administration of SARS-CoV-2 spike protein S1 subunit (S1 protein) to mouse hippocampus induced cognitive deficit and anxiety-like behavior in vivo. These neurological symptoms were accompanied by neuronal cell death in the dorsal and ventral hippocampus as well as glial cell activation. Interestingly, the S1 protein did not directly induce hippocampal cell death in vitro. Rather, it exerted neurotoxicity via glial cell activation, partially through interleukin-1β induction. In conclusion, our data suggest a novel pathogenic mechanism for the COVID-19-associated neurological symptoms that involves glia activation and non-cell autonomous hippocampal neuronal death by the brain-infiltrating S1 protein.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

