Overcoming CAR-Mediated CD19 Downmodulation and Leukemia Relapse with T Lymphocytes Secreting Anti-CD19 T-cell Engagers
- PMID: 35362043
- PMCID: PMC7612571
- DOI: 10.1158/2326-6066.CIR-21-0853
Overcoming CAR-Mediated CD19 Downmodulation and Leukemia Relapse with T Lymphocytes Secreting Anti-CD19 T-cell Engagers
Abstract
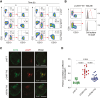
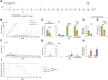
Chimeric antigen receptor (CAR)-modified T cells have revolutionized the treatment of CD19-positive hematologic malignancies. Although anti-CD19 CAR-engineered autologous T cells can induce remission in patients with B-cell acute lymphoblastic leukemia, a large subset relapse, most of them with CD19-positive disease. Therefore, new therapeutic strategies are clearly needed. Here, we report a comprehensive study comparing engineered T cells either expressing a second-generation anti-CD19 CAR (CAR-T19) or secreting a CD19/CD3-targeting bispecific T-cell engager antibody (STAb-T19). We found that STAb-T19 cells are more effective than CAR-T19 cells at inducing cytotoxicity, avoiding leukemia escape in vitro, and preventing relapse in vivo. We observed that leukemia escape in vitro is associated with rapid and drastic CAR-induced internalization of CD19 that is coupled with lysosome-mediated degradation, leading to the emergence of transiently CD19-negative leukemic cells that evade the immune response of engineered CAR-T19 cells. In contrast, engineered STAb-T19 cells induce the formation of canonical immunologic synapses and prevent the CD19 downmodulation observed in anti-CD19 CAR-mediated interactions. Although both strategies show similar efficacy in short-term mouse models, there is a significant difference in a long-term patient-derived xenograft mouse model, where STAb-T19 cells efficiently eradicated leukemia cells, but leukemia relapsed after CAR-T19 therapy. Our findings suggest that the absence of CD19 downmodulation in the STAb-T19 strategy, coupled with the continued antibody secretion, allows an efficient recruitment of the endogenous T-cell pool, resulting in fast and effective elimination of cancer cells that may prevent CD19-positive relapses frequently associated with CAR-T19 therapies.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures



References
-
- Blanco B, Compte M, Lykkemark S, Sanz L, Alvarez-Vallina LT. Cell-redirecting strategies to ‘STAb’ tumors: beyond CARs and bispecific antibodies. Trends Immunol 2019;40:243–57. - PubMed
-
- Romero D. Haematological cancer: blinatumomab facilitates complete responses. Nat Rev Clin Oncol 2018;15:200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

