Ofatumumab with iphosphamide, etoposide and cytarabine for patients with transplantation-ineligible relapsed and refractory diffuse large B-cell lymphoma
- PMID: 35362096
- PMCID: PMC9322457
- DOI: 10.1111/bjh.18166
Ofatumumab with iphosphamide, etoposide and cytarabine for patients with transplantation-ineligible relapsed and refractory diffuse large B-cell lymphoma
Abstract
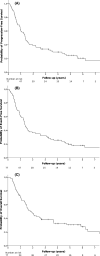
The efficacy of salvage treatment of diffuse large B-cell lymphoma (DLBCL) patients who relapse or progress (rrDLBCL) after initial therapy is limited. Efficacy and safety of ofatumumab with iphosphamide, etoposide and cytarabine (O-IVAC) was evaluated in a single-arm study. Dosing was modified for elderly patients. Patients received up to six cycles of treatment. The primary end-point was the overall response rate (ORR). Patients were evaluated every two cycles and then six and 12 months after treatment. Other end-points included progression-free survival (PFS), event-free survival (EFS), overall survival (OS) and safety. Seventy-seven patients received salvage treatment with O-IVAC. The average age was 56.8 years; 39% had an Eastern Cooperative Oncology Group (ECOG) performance status of at least 3; 78% had disease of Ann Arbor stage 3 or 4; 58% received one or more prior salvage therapies. The ORR for O-IVAC was 54.5%. The median duration of study follow-up was 70 months. The median PFS and EFS were 16.3 months each. The median OS was 22.7 months. Age, ECOG performance status and the number of prior therapy lines were independent predictors of survival. Treatment-related mortality was 15.5%. O-IVAC showed a high response rate in a difficult-to-treat population and is an attractive treatment to bridge to potentially curative therapies.
Keywords: IVAC protocol; ofatumumab; refractory and relapsed diffuse large B-cell lymphoma; salvage treatment.
© 2022 The Authors. British Journal of Haematology published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
Anna Borawska, Andrzej Lange, Agata Malenda,Beata Kumiega, Beata Ostrowska, Grzegorz Rymkiewicz, Jacek Najda, Katarzyna Domańska‐Czyż, Lidia Popławska, Łukasz Targoński, Monika Chełstowska, Michał Osowiecki, Monika Mordak‐Domagała, Monika Świerkowska, Marcin Szymański, Robert Konecki, Tomasz Szpila, Wanda Knopińska‐Posłuszny and Wojciech Michalski have no conflict of interests to declare. Agnieszka Druzd‐Sitek received lecture honoraria from Amgen, Takeda and Celgene‐BMS. Sebastian Giebel received lecture and consulting fees from Gilead, Novartis, Roche and Servier. Andrzej Pluta received consulting fees from Kedrion Pharma. Ewa Paszkiewicz‐Kozik received lecture fees from Roche, Takeda, Abbvie and travel grants from Roche. Jan Maciej Zaucha received consulting fees from Abbvie, Takeda, Roche, Amgen, BMS, Gilead, speaker fees from Novartis, Takeda, Abbvie, Janssen and travel grants from Roche and Gilead. Joanna Romejko‐Jarosińska received lecture honoraria from Roche, Takeda, Abbvie, Gilead and Servier. Jan Walewski received consulting fees from Roche, Takeda, Abbvie, Novartis and Gilead, research funding from Roche and GSK/Novartis and lecture honoraria from Roche, Takeda, Janssen‐Cilag, Servier, Abbvie, Amgen, Novartis and Gilead. Michał Taszner received lecture honoraria from Takeda, Roche and Celgene and travel grants from Novartis.
Figures
References
-
- Pfreundschuh M, Kuhnt E, Trümper L, Osterborg A, Trneny M, Shepherd L, et al. CHOP‐like chemotherapy with or without rituximab in young patients with good‐prognosis diffuse large‐B‐cell lymphoma: 6‐year results of an open‐label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12(11):1013–22. - PubMed
-
- Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long‐term outcome of patients in the LNH‐98.5 trial, the first randomized study comparing rituximab‐CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood. 2010;116(12):2040–5. - PMC - PubMed
-
- Feugier P, Van Hoof A, Sebban C, Solal‐Celigny P, Bouabdallah R, Fermé C, et al. Long‐term results of the R‐CHOP study in the treatment of elderly patients with diffuse large B‐cell lymphoma: a study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 2005;23(18):4117–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources