A new strategy to count and sort neutrophil-derived extracellular vesicles: Validation in infectious disorders
- PMID: 35362257
- PMCID: PMC8971553
- DOI: 10.1002/jev2.12204
A new strategy to count and sort neutrophil-derived extracellular vesicles: Validation in infectious disorders
Abstract
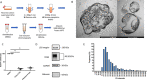
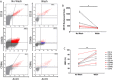
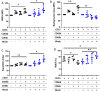
Newly recognized polymorphonuclear neutrophil (PMNs) functions include the ability to release subcellular mediators such as neutrophil-derived extracellular vesicles (NDEVs) involved in immune and thrombo-inflammatory responses. Elevation of their plasmatic level has been reported in a variety of infectious and cardiovascular disorders, but the clinical use of this potential biomarker is hampered by methodological issues. Although flow cytometry (FCM) is currently used to detect NDEVs in the plasma of patients, an extensive characterization of NDEVs has never been done. Moreover, their detection remains challenging because of their small size and low antigen density. Therefore, the objective of the present study was first to establish a surface antigenic signature of NDEVs detectable by FCM and therefore to improve their detection in biological fluids by developing a strategy allowing to overcome their low fluorescent signal and reduce the background noise. By testing a large panel of 54 antibody specificities already reported to be positive on PMNs, we identified a profile of 15 membrane protein markers, including 4 (CD157, CD24, CD65 and CD66c) never described on NDEVs. Among them, CD15, CD66b and CD66c were identified as the most sensitive and specific markers to detect NDEVs by FCM. Using this antigenic signature, we developed a new strategy combining the three best antibodies in a cocktail and reducing the background noise by size exclusion chromatography (SEC). This strategy allowed a significant improvement in NDEVs enumeration in plasma from sepsis patients and made it feasible to efficiently sort NDEVs from COVID-19 patients. Altogether, this work opens the door to a more valuable measurement of NDEVs as a potential biomarker in clinical practice. A similar strategy could also be applied to improve detection by FCM of other rare subpopulations of EVs generated by tissues with limited access, such as vascular endothelium, cancer cells or placenta.
Keywords: EVs sorting; extracellular vesicles; flow cytometry; infectious associated diseases; neutrophils; size exclusion chromatography.
© 2022 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Figures



References
-
- Cointe, S. , Judicone, C. , Robert, S. , Mooberry, M. J. , Poncelet, P. , Wauben, M. , Nieuwland, R. , Key, N. S. , Dignat‐George, F. , & Lacroix, R. (2017). Standardization of microparticle enumeration across different flow cytometry platforms: Results of a multicenter collaborative workshop. Journal of Thrombosis and Haemostasis, 15(1), 187–193. 10.1111/jth.13514 - DOI - PMC - PubMed
-
- Connor, D. E. , Exner, T. , Ma, D. D. F. , & Joseph, J. E. (2010). The majority of circulating platelet‐derived microparticles fail to bind annexin V, lack phospholipid‐dependent procoagulant activity and demonstrate greater expression of glycoprotein Ib. Thrombosis and Haemostasis, 103(5), 1044–1052. 10.1160/TH09-09-0644 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

