Hypnosis to Reduce Distress in Children Undergoing Anorectal Manometry: A Randomized Controlled Pilot Trial
- PMID: 35362456
- PMCID: PMC8978122
- DOI: 10.5056/jnm20274
Hypnosis to Reduce Distress in Children Undergoing Anorectal Manometry: A Randomized Controlled Pilot Trial
Abstract
Background/aims: To assess the effectiveness and feasibility of a brief session of hypnosis to reduce distress in children with functional constipation undergoing anorectal manometry (ARM).
Methods: A partially-blinded randomized controlled pilot trial was conducted in children 4-18 years old scheduled for ARM. Children were randomized to receive a brief session of hypnosis prior to ARM or standard care. Non-blinded and blinded observers rated the child's level of distress using the Observation Scale of Behavioral Distress and a 4-point-Likert scale, respectively. Differences between groups were analyzed using Fisher's exact test or Mann-Whitney U test as appropriate.
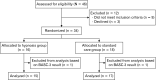
Results: Data from 32 children (15 hypnosis and 17 standard care) were analyzed. Prior to insertion of the catheter, the observed mean levels of distress were lower in the hypnosis group according to both the non-blinded observer (median 0.0 [interquartile range {IQR} 0.0-0.3] vs 1.4 [IQR 0.3-2.4]; P = 0.009) and the blinded observer (median 0.0 [IQR 0.0-0.0] vs 0.5 [IQR 0.0-1.0]; P = 0.044). During ARM, observed and reported levels of distress did not differ significantly. In the hypnosis group, 92.9% of parents and children reported that hypnosis helped the child to relax. There were no significant differences in resting pressure, squeeze pressure, or duration of the procedure between both groups.
Conclusion: A brief session of hypnosis for children before ARM is an easily incorporable intervention that lowers distress levels prior to the procedure and is positively perceived by children and parents.
Keywords: Anxiety; Child; Constipation; Hypnosis; Manometry.
Conflict of interest statement
Figures
Similar articles
-
Hypnosis reduces distress and duration of an invasive medical procedure for children.Pediatrics. 2005 Jan;115(1):e77-85. doi: 10.1542/peds.2004-0818. Pediatrics. 2005. PMID: 15629969 Clinical Trial.
-
Effects of a Hypnosis Session Before General Anesthesia on Postoperative Outcomes in Patients Who Underwent Minor Breast Cancer Surgery: The HYPNOSEIN Randomized Clinical Trial.JAMA Netw Open. 2018 Aug 3;1(4):e181164. doi: 10.1001/jamanetworkopen.2018.1164. JAMA Netw Open. 2018. PMID: 30646110 Free PMC article. Clinical Trial.
-
A randomized clinical trial of a brief hypnosis intervention to control venepuncture-related pain of paediatric cancer patients.Pain. 2009 Apr;142(3):255-263. doi: 10.1016/j.pain.2009.01.017. Epub 2009 Feb 23. Pain. 2009. PMID: 19231082 Clinical Trial.
-
Effectiveness of nonpharmacological interventions to reduce procedural anxiety in children and adolescents undergoing treatment for cancer: A systematic review and meta-analysis.Psychooncology. 2018 Aug;27(8):1889-1899. doi: 10.1002/pon.4749. Epub 2018 May 22. Psychooncology. 2018. PMID: 29714037
-
Anorectal manometry in pediatric settings: A systematic review of 227 studies.Neurogastroenterol Motil. 2021 Apr;33(4):e14006. doi: 10.1111/nmo.14006. Epub 2020 Oct 28. Neurogastroenterol Motil. 2021. PMID: 33118295
Cited by
-
Clinical Hypnosis for Procedural Pain and Distress in Children: A Scoping Review.Pain Med. 2023 Jun 1;24(6):661-702. doi: 10.1093/pm/pnac186. Pain Med. 2023. PMID: 36448690 Free PMC article.
References
LinkOut - more resources
Full Text Sources

