Reactions of a Four-Membered Borete with Carbon, Silicon, and Gallium Donor Ligands: Fused and Spiro-Type Boracycles
- PMID: 35362629
- PMCID: PMC9322404
- DOI: 10.1002/chem.202200673
Reactions of a Four-Membered Borete with Carbon, Silicon, and Gallium Donor Ligands: Fused and Spiro-Type Boracycles
Abstract
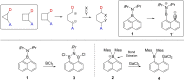
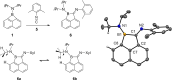
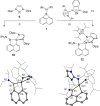
Donor-acceptor cyclopropanes or cyclobutanes are dipolar reagents, which are widely used in the synthesis of complex organic (hetero)cycles in ring expansion reactions. Applying this concept to boron containing heterocycles, the four-membered borete cyclo-iPr2 N-BC10 H6 reacted with the carbon donor ligands 2,6-xylylisonitrile and the carbene IMes :C(NMesCH)2 with ring expansion and ring fusion, respectively. In particular, the tetracyclic structure formed with IMes displays zwitterionic character and absorption in the visible region. In contrast to the carbene IMes, the heavier carbenoids :Si(NDippCH)2 and :Ga(AmIm) with a two-coordinate donor atom afford spiro-type bicyclic compounds, which display four-coordinate geometry at silicon or gallium. (TD-)DFT calculations provide deeper insight into the mechanism of formation and the absorption properties of these new compounds.
Keywords: N-heterocyclic carbenes; donor-acceptor cyclobutanes; gallium carbenoids; inorganic polycycles; inorganic spirocycles; ring expansion reactions; silicon carbenoids.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- None
-
- de Meijere A., Angew. Chem. Int. Ed. 1979, 91, 809–826;
- Angew. Chem. 1979, 91, 867–884;
-
- Z. Rappoport, J. F. Liebman, in PATAI′S Chemistry of Functional Groups-The Chemistry of Cyclobutanes, John Wiley & Sons Ltd. 2005, DOI: 10.1002/9780470682531.
-
- Selected Reviews:
-
- Ghosh K., Das S., Org. Biomol. Chem. 2021, 19, 965–982; - PubMed
LinkOut - more resources
Full Text Sources

