Two-year follow-up of infant and maternal outcomes after planned early delivery or expectant management for late preterm pre-eclampsia (PHOENIX): A randomised controlled trial
- PMID: 35362666
- PMCID: PMC9545311
- DOI: 10.1111/1471-0528.17167
Two-year follow-up of infant and maternal outcomes after planned early delivery or expectant management for late preterm pre-eclampsia (PHOENIX): A randomised controlled trial
Abstract
Objective: We evaluated the best time to initiate delivery in late preterm pre-eclampsia in order to optimise long-term infant and maternal outcomes.
Design: Parallel-group, non-masked, randomised controlled trial.
Setting: Forty-six maternity units in the UK.
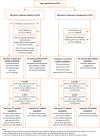
Population: Women with pre-eclampsia between 34+0 and 36+6 weeks of gestation, without severe disease, were randomised to planned delivery or expectant management.
Main outcome measures: Infant neurodevelopmental outcome at 2 years of age, using the Parent Report of Children's Abilities - Revised (PARCA-R) composite score.
Results: Between 29 September 2014 and 10 December 2018, 901 women were enrolled in the trial, with 450 women allocated to planned delivery and 451 women allocated to expectant management. At the 2-year follow-up, the intention-to-treat analysis population included 276 women (290 infants) allocated to planned delivery and 251 women (256 infants) allocated to expectant management. The mean composite standardised PARCA-R scores were 89.5 (SD 18.2) in the planned delivery group and 91.9 (SD 18.4) in the expectant management group, with an adjusted mean difference of -2.4 points (95% CI -5.4 to 0.5 points).
Conclusions: In infants of women with late preterm pre-eclampsia, the average neurodevelopmental assessment at 2 years lies within the normal range, regardless of whether planned delivery or expectant management was pursued. With the lower than anticipated follow-up rate there was limited power to demonstrate that these scores did not differ, but the small between-group difference in PARCA-R scores is unlikely to be clinically important.
Keywords: delivery; infant; neurodevelopment; pre-eclampsia; preterm.
© 2022 The Authors. BJOG: An International Journal of Obstetrics and Gynaecology published by John Wiley & Sons Ltd.
Conflict of interest statement
NM reports personal fees from Shire and Novartis, outside of the submitted work. All other authors report no conflicts of interests. Completed disclosure of interests form available to view online as supporting information.
Figures


Comment in
-
Defer or deliver? Two-year outcomes after late preterm pre-eclampsia.BJOG. 2022 Sep;129(10):1664-1665. doi: 10.1111/1471-0528.17165. Epub 2022 Apr 25. BJOG. 2022. PMID: 35362668 No abstract available.
References
-
- Duley L. The global impact of pre‐eclampsia and eclampsia. Semin Perinatol. 2009;33(3):130–7. - PubMed
-
- Chappell LC, Cluver CA, Kingdom J, Tong S. Pre‐eclampsia. Lancet. 2021;398:341–54. - PubMed
-
- National Institute for Health and Care Excellence . Hypertension in pregnancy: diagnosis and management. 2019. [cited 2022 Jan 26]. Available from: www.nice.org.uk/guidance/ng133 - PubMed
-
- Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018;13:291–310. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

