Quality Standards in State Programs Permitting Cannabis for Medical Uses
- PMID: 35363042
- PMCID: PMC11452082
- DOI: 10.1089/can.2021.0164
Quality Standards in State Programs Permitting Cannabis for Medical Uses
Abstract
Currently in the United States, there exists a patchwork of state-level laws and regulations surrounding cannabis use. Although cannabis (excluding hemp under the Agricultural Improvement Act of 2018, Public Law 115-334) is illegal at the federal level and is not FDA (U.S. Food and Drug Administration) approved for any indication, many states allow patients with qualifying conditions to register for their medical cannabis program (MCP). To better understand the quality of cannabis found in these programs, we collected laws, regulations, and guidance documents available on public state-run websites and compared them with current good manufacturing practices (CGMPs) applicable to finished drug products. CGMPs for human drugs contain minimum requirements for the methods, facilities, and controls used in manufacturing, processing, and packaging of a drug product to assure it is safe for use. Such a comparison will aid the development of consistent quality standards that could, in turn, improve the quality of a wide range of cannabis medical products in development that may be sold in the United States. States may likewise choose to have the cannabis and cannabis-derived products that fall within their MCP to follow quality-focused guidelines, such as those listed in CGMPs, to ensure the quality of these products and promote public health. This study further solidifies the need for standardized testing protocols and methodologies to keep consumers safe.
Keywords: cannabis; government; medical; quality; regulations; testing.
Figures

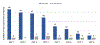


References
-
- Cannabis, Coca, & Poppy: Nature’s Addictive Plants. Cannabis: History. https://museum.dea.gov/exhibits/online-exhibits/cannabis-coca-and-poppy-... Accessed February 23, 2022.
-
- MPP. Medical Marijuana Patient Numbers. 2021. https://www.mpp.org/issues/medical-marijuana/state-by-state-medical-mari... Accessed October 7, 2021.
-
- American for Safe Access. State and Territory Qualifying Condition Chart. 2021. https://www.safeaccessnow.org/condition Accessed October 7, 2021.
-
- U.S. Food and Drug Administration. Electronic Regulatory Submission and Review. 2019. https://www.fda.gov/drugs/forms-submission-requirements/electronic-regul... Accessed October 7, 2021.
-
- U.S. Food and Drug Administration. Center for Drug Evaluation and Research ∣ CDER. 2020. https://www.fda.gov/about-fda/fda-organization/center-drug-evaluation-an... Accessed October 7, 2021.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

