A Comparison of Postprandial Glucose Control in the Medtronic Advanced Hybrid Closed-Loop System Versus 670G
- PMID: 35363054
- PMCID: PMC9353997
- DOI: 10.1089/dia.2021.0568
A Comparison of Postprandial Glucose Control in the Medtronic Advanced Hybrid Closed-Loop System Versus 670G
Abstract
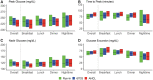
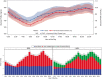
Background: We recently reported that use of an "advanced" hybrid closed-loop system reduced hyperglycemia without increasing hypoglycemia compared to a first-generation system. The aim of this analysis was to evaluate whether this improved performance was specifically related to better mealtime glycemic control. Methods: We conducted a secondary analysis of postprandial glycemic control in an open-label, multinational, randomized crossover trial of 112 participants with type 1 diabetes, aged 14-29, of the Medtronic MiniMed™ 670G hybrid closed-loop system (670G) versus the Medtronic advanced hybrid closed-loop (AHCL) system, for 12 weeks each. We compared glycemic and insulin delivery metrics over a 3 h horizon across all meals to assess system performance and outcomes. Results: Overall meal size and premeal insulin on board were similar during run-in and between 670G and AHCL arms. Compared with 670G arm, premeal, peak, and mean glucose levels were numerically lower in the AHCL arm (167 ± 23, 231 ± 23, and 177 ± 20 mg/dL vs. 175 ± 23, 235 ± 23, and 180 ± 19 mg/dL, respectively), with a trend to lower hyperglycemia level 2 in AHCL arm. Adjusting for premeal glucose level, all postmeal outcomes between 670G and AHCL were statistically similar. Prandial insulin delivery also was similar in both treatment arms (21 ± 9 vs. 23 ± 10 U), with a shift in basal/bolus ratio from 28%/71% in 670G arm to 20%/80% in AHCL arm. Conclusions: Reduced hyperglycemia with AHCL compared to 670G was not related to early postprandial glycemic excursions after adjusting for premeal glucose level (<3 h after meal), but likely to later (>3 h) postprandial or overnight improvements. Further refinements to mealtime bolus algorithms and strategies may more optimally control prandial glycemic excursions.
Keywords: Adolescents; Closed-loop system; Postprandial blood glucose; Young adults.
Conflict of interest statement
Medtronic MiniMed, Inc., provided the MiniMed 670G and AHCL systems, and infusion sets, used in the study. Medtronic, Inc.; provided study continuous glucose monitoring devices and sensors. S.A.W. reports grants from the National Institutes of Health, during the conduct of the study; personal fees from Medtronic, Insulet, Tandem, Eli Lilly, Sanofi, and Zealand, outside the submitted work. R.J.B. reports no conflicts of interest. R.M.B. reports grants and other from Abbott Diabetes Care, grants and other from Eli Lilly, outside the submitted work. R.N. reports grants from National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, nonfinancial support from Medtronic MiniMed, Inc., during the conduct of the study; grants, personal fees and other from DreaMed Diabetes Ltd., grants and nonfinancial support from Medtronic MiniMed, Inc., nonfinancial support from Dexcom, nonfinancial support from Insulet, grants, and personal fees from Eli Lilly, grants and personal fees from NovoNordisk, outside the submitted work. R.W.B. reports grants from National Institutes of Health, nonfinancial support from Medtronic during the conduct of the study; grants, nonfinancial support and study supplies from Dexcom, consulting fees paid to nonprofit employer from Bigfoot Biomedical, outside the submitted work. D.S. reports grants from the National Institutes of Health, during the conduct of the study; grants from the Leona M. and Harry B. Helmsley Charitable Trust, outside the submitted work. L.A.-O. reports no conflicts of interest. D.S.S. reports no conflicts of interest. T.v.d.B. reports grants and fees from NovoNordisk, Medtronic, Sanofi and Ypsomed outside the submitted work. J.S. reports no conflicts of interest. M.L.J. reports grants from the National Institutes of Health during the conduct of the study; grants from Abbott, Dexcom, Medtronic, Eli Lilly, NovoNordisk, Calibra, Insulet, Sanofi, the Leona M. and Harry B. Helmsley Charitable Trust,, and the Juvenile Diabetes Research Foundation, outside the submitted work. P.C. reports personal fees from Dexcom outside the submitted work. M.P. reports grants from the National Institutes of Health via Health Partners, during the conduct of the study; grants from Insulet, Dexcom, Medtronic, Roche, Eli Lilly, Lexicon, OPKO, and AstraZeneca, grants and personal fees from Novo Nordisk and Sanofi, grants, personal fees and owning stock from DreaMed Diabetes, personal fees from RSP Systems and Qulab Medical, grants and personal fees from Pfizer, grants and owning stock from Nutriteen Professional and NG Solutions, outside the submitted work. In addition, M.P. has a patent WO2010097796 issued to DreaMed Diabetes, a patent WO2011039741 issued to DraeMed Diabetes, and a patent WO2019077482 pending.
Figures
Similar articles
-
A comparison of two hybrid closed-loop systems in adolescents and young adults with type 1 diabetes (FLAIR): a multicentre, randomised, crossover trial.Lancet. 2021 Jan 16;397(10270):208-219. doi: 10.1016/S0140-6736(20)32514-9. Lancet. 2021. PMID: 33453783 Free PMC article. Clinical Trial.
-
Improved Glycemic Outcomes With Medtronic MiniMed Advanced Hybrid Closed-Loop Delivery: Results From a Randomized Crossover Trial Comparing Automated Insulin Delivery With Predictive Low Glucose Suspend in People With Type 1 Diabetes.Diabetes Care. 2021 Apr;44(4):969-975. doi: 10.2337/dc20-2250. Epub 2021 Feb 12. Diabetes Care. 2021. PMID: 33579715 Clinical Trial.
-
Feasibility Study of a Hybrid Closed-Loop System with Automated Insulin Correction Boluses.Diabetes Technol Ther. 2021 Apr;23(4):268-276. doi: 10.1089/dia.2020.0448. Epub 2020 Dec 8. Diabetes Technol Ther. 2021. PMID: 33185480
-
Commercial hybrid closed-loop systems available for a patient with type 1 diabetes in 2022.Pediatr Endocrinol Diabetes Metab. 2023;29(1):30-36. doi: 10.5114/pedm.2023.126359. Pediatr Endocrinol Diabetes Metab. 2023. PMID: 37218723 Free PMC article. Review.
-
Where Do We Stand with Closed-Loop Systems and Their Challenges?Diabetes Technol Ther. 2020 Jul;22(7):485-491. doi: 10.1089/dia.2019.0469. Epub 2020 May 22. Diabetes Technol Ther. 2020. PMID: 32069100 Free PMC article. Review.
Cited by
-
2025 Clinical Practice Guidelines for Diabetes Management in Korea: Recommendation of the Korean Diabetes Association.Diabetes Metab J. 2025 Jul;49(4):582-783. doi: 10.4093/dmj.2025.0469. Epub 2025 Jul 1. Diabetes Metab J. 2025. PMID: 40631460 Free PMC article. No abstract available.
-
Hybrid closed-loop systems for managing blood glucose levels in type 1 diabetes: a systematic review and economic modelling.Health Technol Assess. 2024 Dec;28(80):1-190. doi: 10.3310/JYPL3536. Health Technol Assess. 2024. PMID: 39673446 Free PMC article.
-
Early Stages of Automated Insulin Delivery.J Diabetes Sci Technol. 2025 Jul;19(4):908-923. doi: 10.1177/19322968251335685. Epub 2025 Jul 1. J Diabetes Sci Technol. 2025. PMID: 40590463 Free PMC article. Review.
-
New therapies towards a better glycemic control in youths with type 1 diabetes.Pharmacol Res. 2023 Sep;195:106882. doi: 10.1016/j.phrs.2023.106882. Epub 2023 Aug 3. Pharmacol Res. 2023. PMID: 37543096 Free PMC article. Review.
-
Prospective Open-Label, Single-Arm, Single-Center Follow-Up Study of the Application of the Advanced Hybrid Closed Loop System in Well-Controlled Children and Adolescents with Type 1 Diabetes.Diabetes Technol Ther. 2022 Nov;24(11):824-831. doi: 10.1089/dia.2022.0148. Epub 2022 Aug 4. Diabetes Technol Ther. 2022. PMID: 35852811 Free PMC article.
References
-
- Bergenstal RM, Garg S, Weinzimer SA, et al. : Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA 2016;316:1407–1408. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical