Obesity-associated Blunted Subcutaneous Adipose Tissue Blood Flow After Meal Improves After Bariatric Surgery
- PMID: 35363252
- PMCID: PMC9202692
- DOI: 10.1210/clinem/dgac191
Obesity-associated Blunted Subcutaneous Adipose Tissue Blood Flow After Meal Improves After Bariatric Surgery
Abstract
Context: Glucose-dependent insulinotropic peptide (GIP) and meal ingestion increase subcutaneous adipose tissue (SAT) perfusion in healthy individuals. The effects of GIP and a meal on visceral adipose tissue (VAT) perfusion are unclear.
Objective: Our aim was to investigate the effects of meal and GIP on VAT and SAT perfusion in obese individuals with type 2 diabetes mellitus (T2DM) before and after bariatric surgery.
Methods: We recruited 10 obese individuals with T2DM scheduled for bariatric surgery and 10 control individuals. Participants were studied under 2 stimulations: meal ingestion and GIP infusion. SAT and VAT perfusion was measured using 15O-H2O positron emission tomography-magnetic resonance imaging at 3 time points: baseline, 20 minutes, and 50 minutes after the start of stimulation. Obese individuals were studied before and after bariatric surgery.
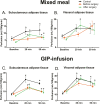
Results: Before bariatric surgery the responses of SAT perfusion to meal (P = .04) and GIP-infusion (P = .002) were blunted in the obese participants compared to controls. VAT perfusion response did not differ between obese and control individuals after a meal or GIP infusion. After bariatric surgery SAT perfusion response to a meal was similar to that of controls. SAT perfusion response to GIP administration remained lower in the operated-on than control participants. There was no change in VAT perfusion response after bariatric surgery.
Conclusion: The vasodilating effects of GIP and meal are blunted in SAT but not in VAT in obese individuals with T2DM. Bariatric surgery improves the effects of a meal on SAT perfusion, but not the effects of GIP. Postprandial increase in SAT perfusion after bariatric surgery seems to be regulated in a GIP-independent manner.
Trial registration: ClinicalTrials.gov NCT01880827.
Keywords: adipose tissue; bariatric surgery; blood flow; glucose-dependent insulinotropic polypeptide; positron emission tomography; type 2 diabetes.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Endocrine Society.
Figures

References
-
- Virtanen KA, Iozzo P, Hällsten K, et al. . Increased fat mass compensates for insulin resistance in abdominal obesity and type 2 diabetes: a positron-emitting tomography study. Diabetes. 2005;54(9):2720-2726. - PubMed
-
- Buchwald H, Estok R, Fahrbach K, et al. . Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248-256.e5. - PubMed
-
- Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3(3):153-165. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

