Expression of Toll-like receptors 3, 7, 9 and cytokines in feline infectious peritonitis virus-infected CRFK cells and feline peripheral monocytes
- PMID: 35363438
- PMCID: PMC8977543
- DOI: 10.4142/jvs.21225
Expression of Toll-like receptors 3, 7, 9 and cytokines in feline infectious peritonitis virus-infected CRFK cells and feline peripheral monocytes
Abstract
Background: The role of Toll-like receptors (TLRs) in a feline infectious peritonitis virus (FIPV) infection is not completely understood.
Objectives: This study examined the expression of TLR3, TLR7, TLR9, tumor necrosis factor-alpha (TNF-α), interferon (IFN)-β, and interleukin (IL)-10 upon an FIPV infection in Crandell-Reese feline kidney (CRFK) cells and feline monocytes.
Methods: CRFK cells and monocytes from feline coronavirus (FCoV)-seronegative cats and FCoV-seropositive cats were infected with type II FIPV-79-1146. At four, 12, and 24 hours post-infection (hpi), the expression of TLR3, TLR7, TLR9, TNF-α, IFN-β, and IL-10, and the viral load were measured using reverse transcription quantitative polymerase chain reaction. Viral protein production was confirmed using immunofluorescence.
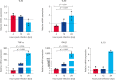
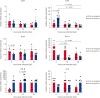
Results: FIPV-infected CRFK showed the upregulation of TLR9, TNF-α, and IFN-β expression between 4 and 24 hpi. Uninfected monocytes from FCoV-seropositive cats showed lower TLR3 and TLR9 expression but higher TLR7 expression compared to uninfected monocytes from FCoV-seronegative cats. FIPV-infected monocytes from FCoV-seropositive cats downregulated TLR7 and TNF-α expression between 4 and 24 hpi, and 4 and 12 hpi, respectively. IFN-β was upregulated early in FIPV-infected monocytes from FCoV-seropositive cats, with a significant difference observed at 12 hpi compared to FCoV-seronegative cats. The viral load in the CRFK and FIPV-infected monocytes in both cohorts of cats was similar over time.
Conclusion: TLR7 may be the key TLR involved in evading the innate response against inhibiting TNF-α production. Distinct TLR expression profiles between FCoV-seronegative and FCoV-seropositive cats were observed. The associated TLR that plays a role in the induction of IFN-β needs to be explored further.
Keywords: Feline infectious peritonitis (FIP); Toll-like receptor (TLR); cytokine; monocytes.
© 2022 The Korean Society of Veterinary Science.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
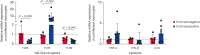
References
-
- Addie DD, Schaap IA, Nicolson L, Jarrett O. Persistence and transmission of natural type I feline coronavirus infection. J Gen Virol. 2003;84(Pt 10):2735–2744. - PubMed
-
- Addie DD, Toth S, Murray GD, Jarrett O. Risk of feline infectious peritonitis in cats naturally infected with feline coronavirus. Am J Vet Res. 1995;56(4):429–434. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

