Phase II Study of Selumetinib in Children and Young Adults With Tumors Harboring Activating Mitogen-Activated Protein Kinase Pathway Genetic Alterations: Arm E of the NCI-COG Pediatric MATCH Trial
- PMID: 35363510
- PMCID: PMC9273373
- DOI: 10.1200/JCO.21.02840
Phase II Study of Selumetinib in Children and Young Adults With Tumors Harboring Activating Mitogen-Activated Protein Kinase Pathway Genetic Alterations: Arm E of the NCI-COG Pediatric MATCH Trial
Abstract
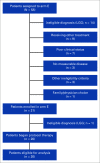
Purpose: The NCI-COG Pediatric MATCH trial assigns patients age 1-21 years with relapsed or refractory solid tumors, lymphomas, and histiocytic disorders to phase II studies of molecularly targeted therapies on the basis of detection of predefined genetic alterations. Patients with tumors harboring mutations or fusions driving activation of the mitogen-activated protein kinase (MAPK) pathway were treated with the MEK inhibitor selumetinib.
Methods: Patients received selumetinib twice daily for 28-day cycles until disease progression or intolerable toxicity. The primary end point was objective response rate; secondary end points included progression-free survival and tolerability of selumetinib.
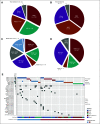
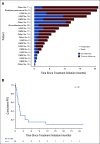
Results: Twenty patients (median age: 14 years) were treated. All were evaluable for response and toxicities. The most frequent diagnoses were high-grade glioma (HGG; n = 7) and rhabdomyosarcoma (n = 7). Twenty-one actionable mutations were detected: hotspot mutations in KRAS (n = 8), NRAS (n = 3), and HRAS (n = 1), inactivating mutations in NF1 (n = 7), and BRAF V600E (n = 2). No objective responses were observed. Three patients had a best response of stable disease including two patients with HGG (NF1 mutation, six cycles; KRAS mutation, 12 cycles). Six-month progression-free survival was 15% (95% CI, 4 to 34). Five patients (25%) experienced a grade 3 or higher adverse event that was possibly or probably attributable to study drug.
Conclusion: A national histology-agnostic molecular screening strategy was effective at identifying children and young adults eligible for treatment with selumetinib in the first Pediatric MATCH treatment arm to be completed. MEK inhibitors have demonstrated promising responses in some pediatric tumors (eg, low-grade glioma and plexiform neurofibroma). However, selumetinib in this cohort with treatment-refractory tumors harboring MAPK alterations demonstrated limited efficacy, indicating that pathway mutation status alone is insufficient to predict response to selumetinib monotherapy for pediatric cancers.
Trial registration: ClinicalTrials.gov NCT03213691.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

