Deubiquitinase CYLD acts as a negative regulator of dopamine neuron survival in Parkinson's disease
- PMID: 35363524
- PMCID: PMC10938605
- DOI: 10.1126/sciadv.abh1824
Deubiquitinase CYLD acts as a negative regulator of dopamine neuron survival in Parkinson's disease
Abstract
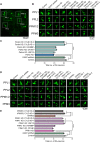
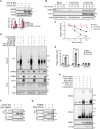
Mutations in PINK1 and parkin highlight the mitochondrial axis of Parkinson's disease (PD) pathogenesis. PINK1/parkin regulation of the transcriptional repressor PARIS bears direct relevance to dopamine neuron survival through augmentation of PGC-1α-dependent mitochondrial biogenesis. Notably, knockout of PARIS attenuates dopaminergic neurodegeneration in mouse models, indicating that interventions that prevent dopaminergic accumulation of PARIS could have therapeutic potential in PD. To this end, we have identified the deubiquitinase cylindromatosis (CYLD) to be a regulator of PARIS protein stability and proteasomal degradation via the PINK1/parkin pathway. Knockdown of CYLD in multiple models of PINK1 or parkin inactivation attenuates PARIS accumulation by modulating its ubiquitination levels and relieving its repressive effect on PGC-1α to promote mitochondrial biogenesis. Together, our studies identify CYLD as a negative regulator of dopamine neuron survival, and inhibition of CYLD may potentially be beneficial in PD by lowering PARIS levels and promoting mitochondrial biogenesis.
Figures





References
-
- Kitada T., Asakawa S., Hattori N., Matsumine H., Yamamura Y., Minoshima S., Yokochi M., Mizuno Y., Shimizu N., Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392, 605–608 (1998). - PubMed
-
- Valente E. M., Abou-Sleiman P. M., Caputo V., Muqit M. M., Harvey K., Gispert S., Ali Z., Del Turco D., Bentivoglio A. R., Healy D. G., Albanese A., Nussbaum R., Gonzalez-Maldonado R., Deller T., Salvi S., Cortelli P., Gilks W. P., Latchman D. S., Harvey R. J., Dallapiccola B., Auburger G., Wood N. W., Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science 304, 1158–1160 (2004). - PubMed
-
- Koyano F., Okatsu K., Kosako H., Tamura Y., Go E., Kimura M., Kimura Y., Tsuchiya H., Yoshihara H., Hirokawa T., Endo T., Fon E. A., Trempe J. F., Saeki Y., Tanaka K., Matsuda N., Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature 510, 162–166 (2014). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

