Neuroimmune Interactions in Peripheral Organs
- PMID: 35363534
- PMCID: PMC9436268
- DOI: 10.1146/annurev-neuro-111020-105359
Neuroimmune Interactions in Peripheral Organs
Abstract
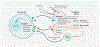
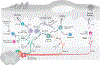
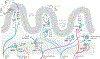
Interactions between the nervous and immune systems were recognized long ago, but recent studies show that this crosstalk occurs more frequently than was previously appreciated. Moreover, technological advances have enabled the identification of the molecular mediators and receptors that enable the interaction between these two complex systems and provide new insights on the role of neuroimmune crosstalk in organismal physiology. Most neuroimmune interactions occur at discrete anatomical locations in which neurons and immune cells colocalize. Here, we describe the interactions of the different branches of the peripheral nervous system with immune cells in various organs, including the skin, intestine, lung, and adipose tissue. We highlight how neuroimmune crosstalk orchestrates physiological processes such as host defense, tissue repair, metabolism, and thermogenesis. Unraveling these intricate relationships is invaluable to explore the therapeutic potential of neuroimmune interactions.
Keywords: mucosal immunology; neuroimmune interactions; neuroimmunology.
Conflict of interest statement
DISCLOSURE STATEMENT
I.M.C. is on scientific advisory boards for GSK Pharmaceuticals and LiMM Therapeutics. His lab receives funding from Allergan Pharmaceuticals for sponsored research. H.V.-F. is on the board of LiMM Therapeutics.
Figures

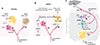
References
-
- Afan AM, Broome CS, Nicholls SE, Whetton AD, Miyan JA. 1997. Bone marrow innervation regulates cellular retention in the murine haemopoietic system. Br. J. Haematol. 98(3):569–77 - PubMed
-
- Andersson RG, Grundström N. 1987. Innervation of airway smooth muscle. Efferent mechanisms. Pharmacol. Ther. 32(2):107–30 - PubMed
-
- Arranz L, Sánchez-Aguilera A, Martín-Pérez D, Isern J, Langa X, et al. 2014. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature 512(7512):78–81 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

