Pathogenicity and virulence of Marburg virus
- PMID: 35363588
- PMCID: PMC8986239
- DOI: 10.1080/21505594.2022.2054760
Pathogenicity and virulence of Marburg virus
Abstract
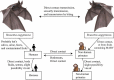
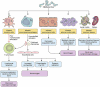
Marburg virus (MARV) has been a major concern since 1967, with two major outbreaks occurring in 1998 and 2004. Infection from MARV results in severe hemorrhagic fever, causing organ dysfunction and death. Exposure to fruit bats in caves and mines, and human-to-human transmission had major roles in the amplification of MARV outbreaks in African countries. The high fatality rate of up to 90% demands the broad study of MARV diseases (MVD) that correspond with MARV infection. Since large outbreaks are rare for MARV, clinical investigations are often inadequate for providing the substantial data necessary to determine the treatment of MARV disease. Therefore, an overall review may contribute to minimizing the limitations associated with future medical research and improve the clinical management of MVD. In this review, we sought to analyze and amalgamate significant information regarding MARV disease epidemics, pathophysiology, and management approaches to provide a better understanding of this deadly virus and the associated infection.
Keywords: Marburg virus; cellular tropism; epidemiology; pathogenicity; transmission dynamics; virulence.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
-
- Kuhn JH, et al. Family - filoviridae. In: King AMQ editor. Virus taxonomy. San Diego: Elsevier; 2012. pp. 665–671.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
