Nepetin reduces virulence factors expression by targeting ClpP against MRSA-induced pneumonia infection
- PMID: 35363605
- PMCID: PMC8986306
- DOI: 10.1080/21505594.2022.2051313
Nepetin reduces virulence factors expression by targeting ClpP against MRSA-induced pneumonia infection
Abstract
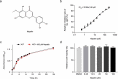
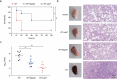
The resistance of Staphylococcus aureus (S. aureus) to various antibiotics has increased dramatically due to the misuse of antibiotics, and thus the development of new anti-infective drugs with new targets is urgently needed to combat resistance. Caseinolytic peptidase P is a case in hydrolase that regulates the virulence level of S. aureus. Here, we found that nepetin, a small-molecule compound from traditional Chinese herbal flavonoids, effectively inhibits ClpP activity. Nepetin suppressed the virulence of S. aureus and effectively combated the lethal pneumonia caused by MRSA. The results of cellular thermal shift assay showed that nepetin could bind to ClpP and reduce the thermal stability of ClpP, and the KD value of 602 nM between them was determined using localized surface plasmon resonance. The binding mode of nepetin and ClpP was further investigated by molecular docking, and it was found that Ser-22 and Gln-47 of ClpP residues were found to be involved in the binding of nepetin to ClpP. In conclusion, we determined that nepetin is a ClpP inhibitor and an effective lead compound for the development of a virulence factor-based treatment for MRSA infection.
Keywords: MRSA; anti-virulence; caseinolytic peptidase P; inhibitor; pneumonia.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
-
- Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339(8):520–532. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
