Early IL-17A production helps establish Mycobacterium intracellulare infection in mice
- PMID: 35363832
- PMCID: PMC9007361
- DOI: 10.1371/journal.ppat.1010454
Early IL-17A production helps establish Mycobacterium intracellulare infection in mice
Abstract
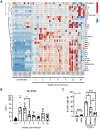
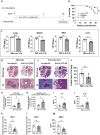
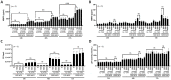
Nontuberculous mycobacteria (NTM) infection is common in patients with structural lung damage. To address how NTM infection is established and causes lung damage, we established an NTM mouse model by intranasal inoculation of clinical isolates of M. intracellulare. During the 39-week course of infection, the bacteria persistently grew in the lung and caused progressive granulomatous and fibrotic lung damage with mortality exceeding 50%. Lung neutrophils were significantly increased at 1 week postinfection, reduced at 2 weeks postinfection and increased again at 39 weeks postinfection. IL-17A was increased in the lungs at 1-2 weeks of infection and reduced at 3 weeks postinfection. Depletion of neutrophils during early (0-2 weeks) and late (32-34 weeks) infection had no effect on mortality or lung damage in chronically infected mice. However, neutralization of IL-17A during early infection significantly reduced bacterial burden, fibrotic lung damage, and mortality in chronically infected mice. Since it is known that IL-17A regulates matrix metalloproteinases (MMPs) and that MMPs contribute to the pathogenesis of pulmonary fibrosis, we determined the levels of MMPs in the lungs of M. intracellulare-infected mice. Interestingly, MMP-3 was significantly reduced by anti-IL-17A neutralizing antibody. Moreover, in vitro data showed that exogenous IL-17A exaggerated the production of MMP-3 by lung epithelial cells upon M. intracellulare infection. Collectively, our findings suggest that early IL-17A production precedes and promotes organized pulmonary M. intracellulare infection in mice, at least in part through MMP-3 production.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

