Prophylactic cyclo-oxygenase inhibitor drugs for the prevention of morbidity and mortality in preterm infants: a network meta-analysis
- PMID: 35363893
- PMCID: PMC8974932
- DOI: 10.1002/14651858.CD013846.pub2
Prophylactic cyclo-oxygenase inhibitor drugs for the prevention of morbidity and mortality in preterm infants: a network meta-analysis
Abstract
Background: Patent ductus arteriosus (PDA) is associated with significant morbidity and mortality in preterm infants. Cyclooxygenase inhibitors (COX-I) may prevent PDA-related complications. Controversy exists on which COX-I drug is the most effective and has the best safety profile in preterm infants.
Objectives: To compare the effectiveness and safety of prophylactic COX-I drugs and 'no COXI prophylaxis' in preterm infants using a Bayesian network meta-analysis (NMA).
Search methods: Searches of Cochrane CENTRAL via Wiley, OVID MEDLINE and Embase via Elsevier were conducted on 9 December 2021. We conducted independent searches of clinical trial registries and conference abstracts; and scanned the reference lists of included trials and related systematic reviews.
Selection criteria: We included randomised controlled trials (RCTs) that enrolled preterm or low birth weight infants within the first 72 hours of birth without a prior clinical or echocardiographic diagnosis of PDA and compared prophylactic administration of indomethacin or ibuprofen or acetaminophen versus each other, placebo or no treatment.
Data collection and analysis: We used the standard methods of Cochrane Neonatal. We used the GRADE NMA approach to assess the certainty of evidence derived from the NMA for the following outcomes: severe intraventricular haemorrhage (IVH), mortality, surgical or interventional PDA closure, necrotizing enterocolitis (NEC), gastrointestinal perforation, chronic lung disease (CLD) and cerebral palsy (CP).
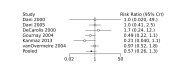
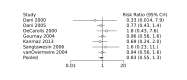
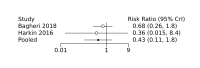
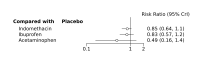
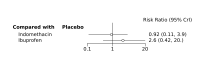
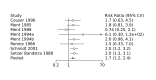
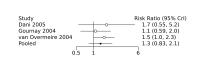
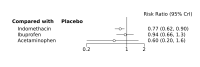
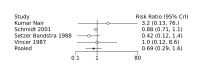
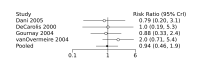
Main results: We included 28 RCTs (3999 preterm infants). Nineteen RCTs (n = 2877) compared prophylactic indomethacin versus placebo/no treatment, 7 RCTs (n = 914) compared prophylactic ibuprofen versus placebo/no treatment and 2 RCTs (n = 208) compared prophylactic acetaminophen versus placebo/no treatment. Nine RCTs were judged to have high risk of bias in one or more domains.We identified two ongoing trials on prophylactic acetaminophen. Bayesian random-effects NMA demonstrated that prophylactic indomethacin probably led to a small reduction in severe IVH (network RR 0.66, 95% Credible Intervals [CrI] 0.49 to 0.87; absolute risk difference [ARD] 43 fewer [95% CrI, 65 fewer to 16 fewer] per 1000; median rank 2, 95% CrI 1-3; moderate-certainty), a moderate reduction in mortality (network RR 0.85, 95% CrI 0.64 to 1.1; ARD 24 fewer [95% CrI, 58 fewer to 16 more] per 1000; median rank 2, 95% CrI 1-4; moderate-certainty) and surgical PDA closure (network RR 0.40, 95% CrI 0.14 to 0.66; ARD 52 fewer [95% CrI, 75 fewer to 30 fewer] per 1000; median rank 2, 95% CrI 1-2; moderate-certainty) compared to placebo. Prophylactic indomethacin resulted in trivial difference in NEC (network RR 0.76, 95% CrI 0.35 to 1.2; ARD 16 fewer [95% CrI, 42 fewer to 13 more] per 1000; median rank 2, 95% CrI 1-3; high-certainty), gastrointestinal perforation (network RR 0.92, 95% CrI 0.11 to 3.9; ARD 4 fewer [95% CrI, 42 fewer to 137 more] per 1000; median rank 1, 95% CrI 1-3; moderate-certainty) or CP (network RR 0.97, 95% CrI 0.44 to 2.1; ARD 3 fewer [95% CrI, 62 fewer to 121 more] per 1000; median rank 2, 95% CrI 1-3; low-certainty) and may result in a small increase in CLD (network RR 1.10, 95% CrI 0.93 to 1.3; ARD 36 more [95% CrI, 25 fewer to 108 more] per 1000; median rank 3, 95% CrI 1-3; low-certainty). Prophylactic ibuprofen probably led to a small reduction in severe IVH (network RR 0.69, 95% CrI 0.41 to 1.14; ARD 39 fewer [95% CrI, 75 fewer to 18 more] per 1000; median rank 2, 95% CrI 1-4; moderate-certainty) and moderate reduction in surgical PDA closure (network RR 0.24, 95% CrI 0.06 to 0.64; ARD 66 fewer [95% CrI, from 82 fewer to 31 fewer] per 1000; median rank 1, 95% CrI 1-2; moderate-certainty) compared to placebo. Prophylactic ibuprofen may result in moderate reduction in mortality (network RR 0.83, 95% CrI 0.57 to 1.2; ARD 27 fewer [95% CrI, from 69 fewer to 32 more] per 1000; median rank 2, 95% CrI 1-4; low-certainty) and leads to trivial difference in NEC (network RR 0.73, 95% CrI 0.31 to 1.4; ARD 18 fewer [95% CrI, from 45 fewer to 26 more] per 1000; median rank 1, 95% CrI 1-3; high-certainty), or CLD (network RR 1.00, 95% CrI 0.83 to 1.3; ARD 0 fewer [95% CrI, from 61 fewer to 108 more] per 1000; median rank 2, 95% CrI 1-3; low-certainty). The evidence is very uncertain on effect of ibuprofen on gastrointestinal perforation (network RR 2.6, 95% CrI 0.42 to 20.0; ARD 76 more [95% CrI, from 27 fewer to 897 more] per 1000; median rank 3, 95% CrI 1-3; very low-certainty). The evidence is very uncertain on the effect of prophylactic acetaminophen on severe IVH (network RR 1.17, 95% CrI 0.04 to 55.2; ARD 22 more [95% CrI, from 122 fewer to 1000 more] per 1000; median rank 4, 95% CrI 1-4; very low-certainty), mortality (network RR 0.49, 95% CrI 0.16 to 1.4; ARD 82 fewer [95% CrI, from 135 fewer to 64 more] per 1000; median rank 1, 95% CrI 1-4; very low-certainty), or CP (network RR 0.36, 95% CrI 0.01 to 6.3; ARD 70 fewer [95% CrI, from 109 fewer to 583 more] per 1000; median rank 1, 95% CrI 1-3; very low-certainty). In summary, based on ranking statistics, both indomethacin and ibuprofen were equally effective (median ranks 2 respectively) in reducing severe IVH and mortality. Ibuprofen (median rank 1) was more effective than indomethacin in reducing surgical PDA ligation (median rank 2). However, no statistically-significant differences were observed between the COX-I drugs for any of the relevant outcomes.
Authors' conclusions: Prophylactic indomethacin probably results in a small reduction in severe IVH and moderate reduction in mortality and surgical PDA closure (moderate-certainty), may result in a small increase in CLD (low-certainty) and results in trivial differences in NEC (high-certainty), gastrointestinal perforation (moderate-certainty) and cerebral palsy (low-certainty). Prophylactic ibuprofen probably results in a small reduction in severe IVH and moderate reduction in surgical PDA closure (moderate-certainty), may result in a moderate reduction in mortality (low-certainty) and trivial differences in CLD (low-certainty) and NEC (high-certainty). The evidence is very uncertain about the effect of acetaminophen on any of the clinically-relevant outcomes.
Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
SM is the principal investigator of a Canadian Institutes of Health Research (CIHR)‐funded prospective study on the relative effectiveness and safety of pharmacotherapeutic agents for treatment of patent ductus arteriosus (PDA) in preterm infants. SM reports working as a neonatologist at a tertiary care neonatal intensive care unit in IWK Health Center (Halifax, Nova Scotia, Canada) where they attend to preterm infants diagnosed with a PDA.
CEG declares no conflict of interest.
AM declares no conflict of interest.
TD declares no conflict of interest.
DMS reports working as a Fellow (Resident PGY6) Neonatal‐Perinatal Medicine at Dalhousie University/IWK Health Center.
MCY declares no conflict of interest.
SK declares no conflict of interest.
BCJ declares no conflict of interest.
JD reports working at IWK Health as Neonatologist and therefore sometimes treat PDAs in preterm infants.
Figures




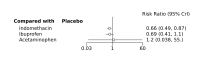






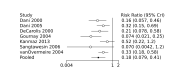

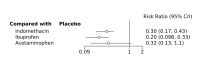
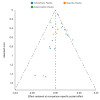
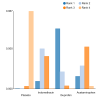


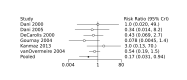
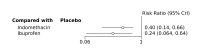
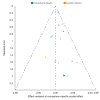



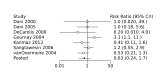

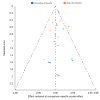
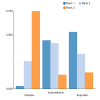

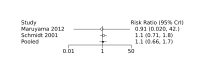
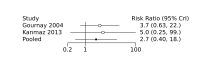
















Update of
References
References to studies included in this review
Bada 1989 {published data only}
-
- Bada HS, Green RS, Pourcyrous M, Leffler CW, Korones SB, Magill HL, et al.Indomethacin reduces the risks of severe intraventricular hemorrhage. Journal of Pediatrics 1989;115(4):631-7. - PubMed
Bagheri 2018 {published data only}
-
- Bagheri M-M, Bahman-Bijari B, Torabi-Nejad M-H, Niknafs P, Mousavi H, Sabzevari F, et al.Is prophylactic parenteral paracetamol effective to diminish incidence of PDA in preterm neonates? A randomized trial. Iranian Journal of Pediatrics 2018;28(4):e11735.
Couser 1996 {published data only}
-
- Couser R J, Ferrara TB, Wright GB, Cabalka AK, Schilling CG, Hoekstra RE, et al.Prophylactic indomethacin therapy in the first twenty-four hours of life for the prevention of patent ductus arteriosus in preterm infants treated prophylactically with surfactant in the delivery room. Journal of Pediatrics 1996;128(5 Pt 1):631-7. - PubMed
-
- Couser RJ, Hoekstra RE, Ferrara B, Wright GB, Cabalka AK, Connet JE.Neurodevelopmental follow-up at 36months’ corrected age of preterm infants treated withprophylactic indomethacin. Archives of Pediatric and Adolescent Medicine 2000;154(6):598-602. - PubMed
Dani 2000 {published data only}
-
- Dani C, Bertini G, Reali M F, Murru P, Fabris C, Vangi V, et al.Prophylaxis of patent ductus arteriosus with ibuprofen in preterm infants. Acta Paediatrica 2000;89(11):1369-74. - PubMed
Dani 2005 {published data only}
-
- Dani C, Bertini G, Pezzati M, Poggi C, Guerrini P, Martano C, et al.Prophylactic ibuprofen for the prevention of intraventricular hemorrhage among preterm infants: a multicenter, randomized study. Pediatrics 2005;115(6):1529-35. - PubMed
De Carolis 2000 {published data only}
-
- De Carolis M P, Romagnoli C, Polimeni V, Piersigilli F, Zecca E, Papacci P, et al.Prophylactic ibuprofen therapy of patent ductus arteriosus in preterm infants. European Journal of Pediatrics 2000;159(5):364-8. - PubMed
Gournay 2004 {published data only}
-
- Gournay V, Roze J C, Kuster A, Daoud P, Cambonie G, Hascoet J M, et al.Prophylactic ibuprofen versus placebo in very premature infants: a randomised, double-blind, placebo-controlled trial. Lancet 2004;364(9449):1939-44. - PubMed
Hanigan 1988 {published data only}
-
- Hanigan WC, Kennedy G, Roemisch F, Anderson R, Cusack T, Powers W.Administration of indomethacin for the prevention of periventricular-intraventricular hemorrhage in high-risk neonates. Journal of Pediatrics 1988;112(6):941-7. - PubMed
Harkin 2016 {published data only}
-
- Harkin P, Harma A, Aikio O, Valkama M, Leskinen M, Saarela T, et al.Paracetamol accelerates closure of the ductus arteriosus after premature birth: a randomized trial. Journal of Pediatrics 2016;177:72-7.e2. - PubMed
-
- Juujärvi S, Kallankari H, Pätsi P, Leskinen M, Saarela T, Hallman M, et al.Follow-up study of the early, randomised paracetamol trial to preterm infants, found no adverse reactions at the two-years corrected age. Acta Paediatrica 2019;108(3):452-8. - PubMed
Jannatdoust 2014 {published data only}
-
- Jannatdoust A, Samadi M, Yeganehdoust S, Heydarzadeh M, Alikhah H, Piri R, et al.Effects of intravenous indomethacin on reduction of symptomatic patent ductus arteriosus cases and decreasing the need for prolonged mechanical ventilation. Journal of Cardiovascular and Thoracic Research 2014;6(4):257-9. - PMC - PubMed
Kanmaz 2013 {published data only}
-
- Kanmaz G, Erdeve O, Canpolat FE, Oğuz SS, Uras N, Altug N, et al.Serum ibuprofen levels of extremely preterm infants treated prophylactically with oral ibuprofen to prevent patent ductus arteriosus. European Journal of Clinical Pharmacology 2013;69(5):1075-81. - PubMed
Krueger 1987 {published data only}
-
- Krueger E, Mellander M, Bratton D, Cotton R.Prevention of symptomatic patent ductus arteriosus with a single dose of indomethacin. Journal of Pediatrics 1987;111(5):749-54. - PubMed
Kumar Nair 2004 {published data only}
-
- Kumar Nair PA, Pai MG, Gazal HA, Da Costa DE, Al Khusaiby SM.Indomethacin prophylaxis for intraventricular hemorrhage in very low birth weight babies. Indian Pediatrics 2004;41(6):551-8. - PubMed
Mahony 1985 {published data only}
-
- Mahony L, Caldwell RL, Girod D A, Hurwitz RA, Jansen RD, Lemons JA, et al.Indomethacin therapy on the first day of life in infants with very low birth weight. Journal of Pediatrics 1985;106(5):801-5. - PubMed
Maruyama 2012 {published data only}
-
- Maruyama K, Fujiu T.Effects of prophylactic indomethacin on renal and intestinal blood flows in premature infants. Pediatrics International 2012;54(4):480-5. - PubMed
Ment 1985 {published data only}
-
- Ment L R, Duncan CC, Ehrenkranz RA, Kleinman CS, Pitt BR, Taylor KJ, et al.Randomized indomethacin trial for prevention of intraventricular hemorrhage in very low birth weight infants. Journal of Pediatrics 1985;107(6):937-43. - PubMed
Ment 1988 {published data only}
-
- Ment LR, Duncan CC, Ehrenkranz RA, Kleinman CS, Taylor K J, Scott DT, et al.Randomized low-dose indomethacin trial for prevention of intraventricular hemorrhage in very low birth weight neonates. Journal of Pediatrics 1988;112(6):948-55. - PubMed
Ment 1994a {published data only}
-
- Ment LR, Oh W, Ehrenkranz RA, Phillip AG, Vohr B, Allan W, et al.Low-dose indomethacin therapy and extension of intraventricular hemorrhage: a multicenter randomized trial. Journal of Pediatrics 1994;124(6):951-5. - PubMed
Ment 1994b {published data only}
-
- Ment LR, Oh W, Ehrenkranz RA, Philip AG, Vohr B, Allan W, et al.Low-dose indomethacin and prevention of intraventricular hemorrhage: a multicenter randomized trial. Pediatrics 1994;93(4):543-50. - PubMed
-
- Ment LR, Vohr B, Allan W, Westerveld M, SparrowSS, Schneider KC, et al.Outcome of children in theindomethacin intraventricular hemorrhage prevention trial. Pediatrics 2000;105:485-91. - PubMed
-
- Ment LR, Vohr B, Oh W, Scott DT, Allan WC, Westerveld M, et al.Neurodevelopmental outcome at 36 months' corrected age of preterm infants in the Multicenter Indomethacin Intraventricular Hemorrhage Prevention Trial. Pediatrics 1996;98:714-8. - PubMed
-
- Ment LR, Vohr BR, Makuch RW, Westerveld M, Katz KH, Schneider KC, et al.Prevention of intraventricular hemorrhage by indomethacin in male preterm infants. Journal of Pediatrics 2004;145(6):832-4. - PubMed
Morales‐Suarez 1994 {published data only}
-
- Morales-Suarez M, De Jesus Sanchez-Gil T, Lemus-Varela L, Udaeta-Mora E.Low doses indomethacin for prevention of intraventricular hemorrhage in the preterm newborn infant with mechanical ventilation: final report of a randomized study [Estudiocomparavito de dosis baja de indometacina profilactica parahemorragia subependimaria/intraventricular en neonatospretermino con ventilation mecanica]. Boletin Medico del Hospital Infantil de Mexico 1994;51(6):389-94.
Rennie 1986 {published data only}
Sangtawesin 2006 {published data only}
-
- Sangtawesin V, Sangtawesin C, Raksasinborisut C, Sathirakul K, Kanjanapattanakul W, Khorana M, et al.Oral ibuprofen prophylaxis for symptomatic patent ductus arteriosus of prematurity. Journal of the Medical Association of Thailand 2006;89(3):314-21. - PubMed
Schmidt 2001 {published data only}
-
- Ohlsson A, Roberts RS, Schmidt B, Davis P, ModdemanD, Saigal S, et al.Male/female differences in indomethacineffects in preterm infants. Journal of Pediatrics 2005;147:860-2. - PubMed
-
- Schmidt B, Davis P, Moddemann D, Ohlsson A, Roberts RS, Saigal S, et al.Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. New England Journal of Medicine 2001;344(26):1966-72. - PubMed
-
- Schmidt B, Roberts RS, Fanaroff A, Davis P, Kirpalani HM, Nwaesei C, et al.Indomethacin prophylaxis, patent ductusarteriosus, and the risk of bronchopulmonary dysplasia:further analyses from the Trial of Indomethacin Prophylaxisin Preterms (TIPP). Journal of Pediatrics 2006;148:730-4. - PubMed
-
- Zupancic JA, Richardson DK, O’Brien BJ, Cronin CG, Schmidt B, Roberts R, et al.Retrospective economic evaluation of a controlled trial of indomethacin prophylaxis for patent ductus arteriosus in premature infants. Early Human Development 2006;82:97-103. - PubMed
Setzer Bandstra 1988 {published data only}
-
- Bandstra ES, Montalvo BM, Goldberg RN, Pacheco I, Ferrer PL, Flynn J, et al.Prophylactic indomethacin for prevention of intraventricular hemorrhage in premature infants. Pediatrics 1988;82(4):533-42. - PubMed
-
- Setzer ES, Morse BM, Goldberg RN, Smith M, Bancalari E.Prophylactic indomethacin and intraventricular hemorrhagein the premature. In: Pediatric Research. Vol. 18. 1984:345A.
-
- Setzer ES, Torres-Arraut E, Gomez-del-Rio M, Young ML, Pacheco I, Ferrer PL, et al.Cardiopulmonary effects of prophylactic indomethacin in the very low birth weight infant. In: Pediatric Research. Vol. 18. 1984:346A.
Supapannachart 1999 {published data only}
-
- Supapannachart S, Khowsathit P, Patchakapati B.Indomethacin prophylaxis for patent ductus arteriosus (PDA) in infants with a birth weight of less than 1250 grams. Journal of the Medical Association of Thailand = Chotmaihet Thangphaet 1999;82 Suppl 1:S87-92. - PubMed
Van Overmeire 2004 {published data only}
-
- Van Overmeire B, Allegaert K, Casaer A, Debauche C, Decaluwé W, Jespers A, et al.Prophylactic ibuprofen in premature infants: a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2004;364(9449):1945-9. - PubMed
-
- Van Overmeire B, Casaer A, Allegaert K, Debauche C, Decaluwe W, Jespers A.Multicenter ibuprofen prophylaxis study (MIPS) in preterm infants: preliminary data. Pediatric Research 2002;52:825.
Vincer 1987 {published data only}
-
- Vincer M, Allen A, Evans J, Nwaesei C, Stinson D, Rees E, et al.Early intravenous indomethacin prolongs respiratory support in very low birth weight infants. Acta Paediatrica 1987;76(6):894-7. - PubMed
-
- Vincer M, Allen A.Does prophylactic indomethacin inVLBW (<1500 grams birth weight) infants cause cerebral palsy (CP)? Pediatric Research 1998;43:232.
Vogtmann 1988 {published data only}
-
- Vogtmann C, Grubbe G, Ruckhäberle KE, Böttcher H, Ockert C.Effects of early therapy with indomethacin on the manifestation of a persistent ductus arteriosus in extremely underweight premature infants [Auswirkungen einer Frühtherapie mit Indomethazin auf die Manifestation eines persistierenden Ductus arteriosus bei extrem untergewichtigen Frühgeborenen]. Monatsschrift Kinderheilkund 1988;136(9):636-9. - PubMed
References to studies excluded from this review
Alfaleh 2008 {published data only}
-
- Alfaleh K, Smyth JA, Roberts RS, Solimano A, Asztalos EV, Schmidt B, Trial of Indomethacin Prophylaxis in Preterms Investigators.Prevention and 18-month outcomes of serious pulmonary hemorrhage in extremely low birth weight infants: results from the trial of indomethacin prophylaxis in preterms. Pediatrics 2008;121(2):e233-8. - PubMed
Barrington 1986 {published data only}
-
- Barrington KJ, Finer NN, Ment LR.Indomethacin for prevention of intraventricular hemorrhage. Journal of Pediatrics 1986;109(2):396-8. - PubMed
Cotts 2009 {published data only}
-
- Cotts T.Escalating dose indomethacin for prophylactic closure of patent ductus arteriosus does not improve closure rates and is associated with increased complications. Journal of Pediatrics 2009;154(1):153. - PubMed
Domanico 1994 {published data only}
-
- Domanico RS, Waldman JD, Lester LA, McPhillips HA, Catrambone JE, Covert RF.Prophylactic indomethacin reduces the incidence of pulmonary hemorrhage and patent ductus arteriosus in surfactant-treated infants. Pediatric Research 1994;35:331A.
Gregoire 2004 {published data only}
-
- Gregoire N, Gualano V, Geneteau A, Millerioux L, Brault M, Mignot A, et al.Population pharmacokinetics of ibuprofen enantiomers in very premature neonates. Journal of Clinical Pharmacology 2004;44(10):1114-24. - PubMed
Gutierrez 1987 {published data only}
-
- Gutierrez NG, Lapasset M.Prophylactic indomethacin and the incidence of patent ductus arteriosus in preterm neonates. Proceedings of the 3rd Argentinian Congress of Perinatology, Buenos Aires 1987;62.
Hammerman 1986 {published data only}
-
- Hammerman C, Strates E, Valaitis S.The silent ductus: its precursors and its aftermath. Pediatric Cardiology 1986;7(3):121-7. - PubMed
Hammerman 2005 {published data only}
-
- Hammerman C, Kaplan M.Prophylactic ibuprofen in premature infants: a multicentre, randomised, double-blind, placebo-controlled trial. Journal of Pediatrics 2005;146(5):709-10. - PubMed
Harma 2018 {published data only}
-
- Harma A, Harkin P, Leskinen M, Valkama M, Saarela T, Aikio O, et al.Does intravenous paracetamol offer neonatal brain protection? Study of hemodynamics and cerebral oxygenation in very premature infants. Neonatology 2018;113(4):417-8. [DOI: ]
Kääpä 1985 {published data only}
-
- Kääpä P.Early closure of ductus arteriosus with indomethacin [Der Frühverschluss des Ductus arteriosus mit Indomethazin]. Klinische Pädiatrie 1985;197(2):172. [DOI: doi: 10.1055/s-2008-1033961] - PubMed
Liebowitz 2017 {published data only}
Mahony 1982 {published data only}
-
- Mahony L, Carnero V, Brett C, Heymann MA, Clyman RI.Prophylactic indomethacin therapy for patent ductus arteriosus in very-low-birth-weight infants. New England Journal of Medicine 1982;306(9):506-10. - PubMed
McGuire 2002 {published data only}
Meau‐Petit 2005 {published data only}
-
- Meau-Petit V.Prophylactic ibuprofen vs placebo in very premature infants: a randomised, double blind, placebo-controlled trial. Archives de Pédiatrie 2005;12:605-8.
Ment 1987 {published data only}
-
- Ment LR.Randomized low-dose indomethacin trial for the prevention of intraventricular hemorrhage in very low birth weight neonates. Annals of Neurology 1987;22(3):406-7. - PubMed
Ment 1998 {published data only}
-
- Ment LR, Westerveld M, Makuch R, Vohr B, Allan WC.Cognitive outcome at 4 1/2 years of very low birth weight infants enrolled in the multicenter indomethacin intraventricular hemorrhage prevention trial. Pediatrics 1998;102(1 Pt 1):159-60. - PubMed
Ment 1999 {published data only}
-
- Ment LR, Vohr B, Allan W, Westerveld M, Katz KH, Schneider KC, et al.The etiology and outcome of cerebral ventriculomegaly at term in very low birth weight preterm infants. Pediatrics 1999;104(2 Pt 1):243-8. - PubMed
Morales‐Suarez 1992 {published data only}
-
- Morales-Suarez M, Lemus-Varela L, Udaeta-Mora E, Cardiel-Marmolejo L, Rodríguez-Balderrama I, Liz-Cedillo RE.Indomethacin in the prevention of subependymal-intraventricular hemorrhage in preterm newborns with conventional mechanical ventilation [Indometacina en la prevención de la hemorragia subependimaria-intraventricular del recién nacido pretérmino con ventilación mecánica convencional]. Boletín Médico del Hospital Infantil de México 1992;49(4):217-24. - PubMed
Naulaers 2005 {published data only}
Pleacher 2004 {published data only}
Puckett 1985 {published data only}
-
- Puckett CG, Cox MA, Haskins KS, Fisher DJ.Prophylactic indomethacin (I) for the prevention of patent ductus arteriosus (PDA). Pediatric Research 1985;19:358.
Roze 2003 {published data only}
-
- Roze JC, Gournay V, Daoud P, Cambonie G, Chamboux C, Hascoet JM, et al.Multicenter double blind randomized placebo-controlled study comparing prophylactic versus curative ibuprofen treatment in premature infants less than 28 weeks of gestation. Pediatric Research 2003;53:386A.
Rubaltelli 1998 {published data only}
-
- Rubaltelli F, Bertini G, Reali MF, Vangi V, Dani C.Does early closure of PDA with ibuprofen reduce the severity of RDS in premature infants? Pediatric Research 1998;43:296A.
Schmidt 2002 {published data only}
-
- Schmidt B, Wright LL, Davis P, Solimano A, Roberts RS, Indomethacin Prophylaxis in Preterms Investigators.Ibuprofen prophylaxis in preterm neonates. Lancet 2002;360(9331):492. - PubMed
Schmidt 2011 {published data only}
-
- Schmidt B, Seshia M, Shankaran S, Mildenhall L, Tyson J, Lui K, et al, Trial of Indomethacin Prophylaxis in Preterms Investigators.Effects of prophylactic indomethacin in extremely low-birth-weight infants with and without adequate exposure to antenatal corticosteroids. Archives of Pediatrics and Adolescent Medicine 2011;165(7):642-6. - PMC - PubMed
Tyson 2002 {published data only}
-
- Tyson JE.Does indomethacin prophylaxis benefit extremely low birth weight infants? Results of a placebo-controlled multicenter trial. Pediatric Research 2002;51(1):1.
Valls‐i‐Soler 1999 {published data only}
-
- Valls-i-Soler A, Lopez-de-Heredia J, Roman L, Fernandez-Ruanova MB.Prophylactic indomethacin (INDO) in very low birth weight (VLBW) infants with RDS. A pilot study on the effects of a slow infusion rate. Pediatric Research 1999;45(6):901.
van Overmeire 2002 {published data only}
-
- Overmeire B, Casaer A, Allegaert K, Debauche C, Decaluwe W, Jespers A, et al.Multicenter randomized double-blind placebo-controlled trial of ibuprofen prophylaxis in very preterm infants. Pediatric Research 2002;51(4):379A.
Varvarigou 1996 {published data only}
-
- Varvarigou A, Bardin CL, Beharry K, Chemtob S, Papageorgiou A, Aranda JV.Early ibuprofen administration to prevent patent ductus arteriosus in premature newborn infants. JAMA 1996;275(7):539-44. [PMID: ] - PubMed
Vohr 1999 {published data only}
-
- Vohr B, Allan WC, Scott DT, Katz KH, Schneider KC, Makuch RW, et al.Early-onset intraventricular hemorrhage in preterm neonates: incidence of neurodevelopmental handicap. Seminars in Perinatology 1999;23(3):212-7. - PubMed
Zarkesh 2013 {published data only}
-
- Zarkesh MR, Nili F, Akbari Asbagh P, Nayeri FS, Naeem A.Prophylactic treatment with oral paracetamol for patent ductus arteriosus in preterm infants admitted in Nicu Vali-Asr Hospital In 2012. Iranian Journal Of Pediatrics 2013;23(Suppl 1):54-5.
References to studies awaiting assessment
Akbari Asbagh 2015 {published data only}
-
- Akbari Asbagh P, Zarkesh MR, Nili F, Nayeri FS, Tofighi Naeem A.Prophylactic teatment with oral paracetamol for patent ductus arteriosus in preterm infants: a randomized clinical trial. Tehran University Medical Journal 2015;73(2):86-92.
Kalani 2016 {published data only}
-
- Kalani M, Shariat M, Khalesi N, Farahani Z, Ahmadi S.A comparison of early ibuprofen and indomethacin administration to prevent intraventricular hemorrhage among preterm infants. Acta Medica Iranica 2016;54(12):788-92. - PubMed
Seok 1998 {published data only}
-
- Seok EJ, Kim EJ, Jeon SS, Seo SS.Effect of indomethacin therapy on prevention of intraventricular hemorrhage in very low birth weight infant. Journal of the Korean Society of Neonatal Science 1998;5(1):27-34.
References to ongoing studies
NCT03641209 {published data only}
-
- Aikio O.Extremely low gestatonal age infants' paracetamol study (Paras) [Extremely low gestational age infants' paracetamol study: a randomized trial]. https://clinicaltrials.gov/ct2/show/NCT03641209 (first received 21 August 2018).
NCT04459117 {published data only}
-
- Rozé JC.Prophylactic treatment of the ductus arteriosus in preterm Infants by acetaminophen (TREOCAPA). https://clinicaltrials.gov/ct2/show/NCT04459117 (first received 7 July 2020).
Additional references
Baik 2015
-
- Baik N, Urlesberger B, Schwaberger B, Schmölzer GM, Avian A, Pichler G.Cerebral haemorrhage in preterm neonates: does cerebral regional oxygen saturation during the immediate transition matter? Archives of Disease in Childhood: Fetal and Neonatal Edition 2015;100(5):F422-7. - PubMed
Baker 2002
Ballabh 2010
Bauer 2013
Bayley 1993
-
- Steiner S.Bayley Scales of Infant Development. 2nd edition. San Antonio, TX: The Psychological Corporation, 1993.
Bayley 2005
-
- Bayley N.Bayley Scales of Infant and Toddler Development. 3rd edition. San Antonio, TX: The Psychological Corporation, 2005.
Bell 1978
Benitz 2016
Bisaglia 2002
-
- Bisaglia M, Venezia V, Piccioli P, Stanzione S, Porcile C, Russo C, et al.Acetaminophen protects hippocampal neurons and PC12 cultures from amyloid beta-peptides induced oxidative stress and reduces NF-kappaB activation. Neurochemistry International 2002;41(1):43-54. - PubMed
Brignardello‐Petersen 2018
-
- Brignardello-Petersen R, Bonner A, Alexander PE, Siemieniuk RA, Furukawa TA, Rochwerg B, et al, GRADE Working Group.Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. Journal of Clinical Epidemiology 2018;93:36-44. [DOI: 10.1016/j.jclinepi.2017.10.005] [PMID: ] - DOI - PubMed
Brignardello‐Petersen 2021
Brown 1979
Chung 2005
-
- Chung M-Y, Fang P-C, Chung C-H, Huang C-B, Ou Yang M-H, Chen C-C.Risk factors for hemodynamically-unrelated cystic periventricular leukomalacia in very low birth weight premature infants. Journal of the Formosan Medical Association 2005;104(8):571-7. [PMID: ] - PubMed
Cimatti 2020
Cipriani 2013
Clyman 2012
Coombs 1991
Costantini 2020
-
- Costantini I, Paul E, Caldwell DM, López-López JA, Pearson RM.Protocol for a systematic review and network meta-analysis of randomised controlled trials examining the effectiveness of early parenting interventions in preventing internalising problems in children and adolescents. Systematic Reviews 2020;9(1):244. - PMC - PubMed
Couser 2000
-
- Couser RJ, Hoekstra RE, Ferrara B, Wright GB, Cabalka AK, Connet JE.Neurodevelopmental follow-up at 36months’ corrected age of preterm infants treated withprophylactic indomethacin. Archives of Pediatric and Adolescent Medicine 2000;154(6):598-602. - PubMed
Covidence [Computer program]
-
- Veritas Health Innovation Covidence.Version accessed 15 September 2018. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
de Vries 1992
Dias 2010
Dias 2013
-
- Dias S, Sutton AJ, Ades AE, Welton NJ.Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Medical Decision Making: an International Journal of the Society for Medical Decision Making 2013;33(5):607-17. [DOI: 10.1177/0272989X12458724] [PMID: ] - DOI - PMC - PubMed
Dice 2007
Dollberg 2005
Donegan 2010
Drougia 2007
Ehrenkranz 2005
-
- Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al, National Institutes of Child Health and Human Development Neonatal Research Network.Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005;116(6):1353-60. [DOI: 10.1542/peds.2005-0249] [PMID: 16322158] - DOI - PubMed
Fowlie 2010
Friedman 1976
Gelman 1992
-
- Gelman A, Rubin DB.Inference from iterative simulation using multiple sequences. Statistical Science 1992;7(4):457-72. [DOI: 10.1214/ss/1177011136] - DOI
Gournay 2011
Grèen 1989
Griffiths 1954
-
- Griffiths R.The Abilities of Babies: a Study of Mental Measurement. London: University of London Press, 1954.
Griffiths 1970
-
- Griffiths R.The Abilities of Young Children: a Comprehensive System of Mental Measurement for the First Eight Years. London: Child Development Research Center, 1970.
Guyatt 2008
-
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al, GRADE Working Group.GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [DOI: 10.1136/bmj.39489.470347.AD] [PMID: ] - DOI - PMC - PubMed
Hamrick 2020
Härkin 2016
-
- Härkin P, Härmä A, Aikio O, Valkama M, Leskinen M, Saarela T, et al.Paracetamol accelerates closure of the ductus arteriosus after premature birth: a randomized trial. Journal of Pediatrics 2016;177:72-7.e2. - PubMed
Härmä 2020
-
- Härmä A, Aikio O, Härkin P, Leskinen M, Valkama M, Saarela T, et al.Subgroup analysis of the early paracetamol trial to preterm infants found haemodynamic changes and improved oxygenation. Early Human Development 2020;145:105042. [PMID: ] - PubMed
Higgins 2019
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors).Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. Chichester (UK): John Wiley & Sons, 2019.
Jacobs 2013
Jain 2015
Ji 2020
-
- Ji Y, Azuine RE, Zhang Y, Hou W, Hong X, Wang G, et al.Association of cord plasma biomarkers of In utero acetaminophen exposure with risk of attention-deficit/hyperactivity disorder and autism spectrum disorder in childhood. JAMA Psychiatry 2020;77(2):180-9. [DOI: 10.1001/jamapsychiatry.2019.3259] [PMID: ] - DOI - PMC - PubMed
Juujärvi 2019
-
- Juujärvi S, Kallankari H, Pätsi P, Leskinen M, Saarela T, Hallman M, et al.Follow-up study of the early, randomised paracetamol trial to preterm infants, found no adverse reactions at the two-years corrected age. Acta Paediatrica, International Journal of Paediatrics 2019;108(3):452-8. - PubMed
Lambert 2005
Le 2015
Lu 2004
Luu 2009
Ment 1992
-
- Ment LR, Stewart WB, Ardito TA, Huang E, Madri JA.Indomethacin promotes germinal matrix microvessel maturation in the newborn beagle pup. Stroke 1992;23(8):1132-7. [PMID: ] - PubMed
Ment 1996
-
- Ment L R, Vohr B, Oh W, Scott D T, Allan W C, Westerveld M, et al.Neurodevelopmental outcome at 36 months' corrected age of preterm infants in the Multicenter Indomethacin Intraventricular Hemorrhage Prevention Trial. Pediatrics 1996;98:714-8. - PubMed
Ment 2000
-
- Ment LR, Vohr B, Allan W, Westerveld M, Sparrow SS, Schneider KC, et al.Outcome of children in theindomethacin intraventricular hemorrhage prevention trial. Pediatrics 2000;105:485-91. - PubMed
Ment 2004
-
- Ment LR, Vohr BR, Makuch RW, Westerveld M, Katz KH, Schneider KC, et al.Prevention of intraventricular hemorrhage by indomethacin in male preterm infants. Journal of Pediatrics 2004;145(6):832-4. - PubMed
Meyer 1991
Mitra 2018
-
- Mitra S, Florez ID, Tamayo ME, Mbuagbaw L, Vanniyasingam T, Veroniki A, et al.Association of placebo, indomethacin, ibuprofen, and acetaminophen with closure of hemodynamically significant patent ductus arteriosus in preterm infants: a systematic review and meta-analysis. JAMA 2018;319(12):1221-38. [DOI: 10.1001/jama.2018.1896] [PMID: ] - DOI - PMC - PubMed
Moher 2009
Ohlsson 1993
Ohlsson 2005
-
- Ohlsson A, Roberts RS, Schmidt B, Davis P, ModdemanD, Saigal S, et al.Male/female differences in indomethacin effects in preterm infants. Journal of Pediatrics 2005;147:860-2. - PubMed
Ohlsson 2020a
Ohlsson 2020b
Ohlsson 2020c
Owen 2019
Palmer 2016
-
- Palmer T, Sterne J, editors.Meta-Analysis in Stata. In: An Updated Collection From the Stata Journal. College Station (TX): Stata Press, 2016.
Papile 1978
Puhan 2014
Pumberger 2002
-
- Pumberger W, Mayr M, Kohlhauser C, Weninger M.Spontaneous localized intestinal perforation in very-low-birth-weight infants: a distinct clinical entity different from necrotizing enterocolitis. Journal of the American College of Surgeons 2002;195(6):796-803. [DOI: 10.1016/s1072-7515(02)01344-3] [PMID: ] - DOI - PubMed
R Core Team 2020 [Computer program]
-
- R Foundation for Statistical Computing R: A language and environment for statistical computing.Vienna, Austria: R Foundation for Statistical Computing, 2020. Available at www.R-project.org.
Reese 2017
Ryan 2019
Salanti 2011
Sangtawesin 2008
-
- Sangtawesin C, Sangtawesin V, Lertsutthiwong W, Kanjanapattanakul W, Khorana M, Ayudhaya JK.Prophylaxis of symptomatic patent ductus arteriosus with oral ibuprofen in very low birth weight infants. Journal of the Medical Association of Thailand 2008;91(Suppl 3):S28-34. - PubMed
Santesso 2020
Schmidt 2006
-
- Schmidt B, Roberts RS, Fanaroff A, Davis P, Kirpalani HM, Nwaesei C, et al.Indomethacin prophylaxis, patent ductusarteriosus, and the risk of bronchopulmonary dysplasia:further analyses from the Trial of Indomethacin Prophylaxisin Preterms (TIPP). Journal of Pediatrics 2006;148:730-4. - PubMed
Schünemann 2013
-
- Schünemann H, Brozek J, Guyatt G, Oxman A, editors.Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Setzer 1984a
-
- Setzer ES, Morse BM, Goldberg RN, Smith M, Bancalari E.Prophylactic indomethacin and intraventricular hemorrhage in the premature. In: Pediatric Research. Vol. 18. 1984:345.
Setzer 1984b
-
- Setzer ES, Torres-Arraut E, Gomez-del-Rio M, Young ML, Pacheco I, Ferrer PL, et al.Cardiopulmonary effects of prophylactic indomethacin in the very low birth weight infant. In: Pediatric Research. Vol. 18. 1984:346.
Seyberth 1983
-
- Seyberth HW, Rascher W, Hackenthal R, Wille L.Effect of prolonged indomethacin therapy on renal function and selected vasoactive hormones in very-low-birth-weight infants with symptomatic patent ductus arteriosus. Journal of Pediatrics 1983;103(6):979-84. [DOI: 10.1016/s0022-3476(83)80736-7] [PMID: ] - DOI - PubMed
Stavel 2017
-
- Stavel M, Wong J, Cieslak Z, Sherlock R, Claveau M, Shah PS.Effect of prophylactic indomethacin administration and early feeding on spontaneous intestinal perforation in extremely low-birth-weight infants. Journal of Perinatology: Official Journal of the California Perinatal Association 2017;37(2):188-93. [DOI: 10.1038/jp.2016.196] [PMID: ] - PubMed
The Canadian Neonatal Network 2019
-
- The Canadian Neonatal Network.CNN 2018 Annual Report. Available at canadianneonatalnetwork.org/portal/Default.aspx (accessed 15 December 2020).
Van Overmeire 2002
-
- Van Overmeire B, Casaer A, Allegaert K, Debauche C, Decaluwe W, Jespers A.Multicenter ibuprofen prophylaxis study (MIPS) in preterm infants: preliminary data. Pediatric Research 2002;52:825.
van Valkenhoef 2012
Vergeade 2016
Veroniki 2013
Vincer 1998
-
- Vincer M, Allen A.Does prophylactic indomethacin inVLBW (<1500 grams birth weight) infants cause cerebral palsy (CP)? Pediatric Research 1998;43:232.
Vohr 2003
-
- Vohr BR, Allan WC, Westerveld M, Schneider KC, Katz KH, Makuch RW, et al.School-age outcomes of very low birth weight infants in the indomethacin intraventricular hemorrhage prevention trial. Pediatrics 2003;111(4 Pt 1):e340-6. - PubMed
White 2012
Wolf 1989
Yepes‐Nuñez 2019
-
- Yepes-Nuñez JJ, Li SA, Guyatt G, Jack SM, Brozek JL, Beyene J, et al.Development of the summary of findings table for network meta-analysis. Journal of Clinical Epidemiology 2019;115:1-13. [10.1016/j.jclinepi.2019.04.018] [PMID: ] - PubMed
Ystrom 2017
Zeng 2021
Zupancic 2006
-
- Zupancic JA, Richardson DK, O’Brien BJ, Cronin CG, Schmidt B, Roberts R, et al.Retrospective economic evaluation of a controlled trial of indomethacin prophylaxis for patent ductus arteriosus in premature infants. Early Human Development 2006;82:97-103. - PubMed
References to other published versions of this review
Mitra 2021
-
- Mitra S, Gardner CE, MacLellan A, Disher T, Styranko DM, Kuhle SJ, et al.Prophylactic cyclo‐oxygenase inhibitor drugs for the prevention of morbidity and mortality in preterm infants: a network meta‐analysis. Cochrane Database of Systematic Reviews 2021, Issue 1. Art. No: CD013846. [DOI: 10.1002/14651858.CD013846] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

