Functional redundancy among Polycomb complexes in maintaining the pluripotent state of embryonic stem cells
- PMID: 35364009
- PMCID: PMC9120860
- DOI: 10.1016/j.stemcr.2022.02.020
Functional redundancy among Polycomb complexes in maintaining the pluripotent state of embryonic stem cells
Abstract
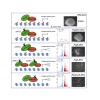
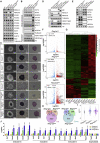
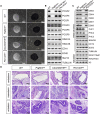
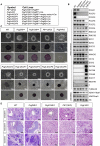
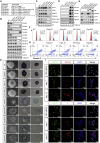
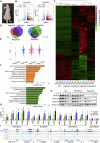
Polycomb group proteins assemble into multi-protein complexes, known as Polycomb repressive complexes 1 and 2 (PRC1 and PRC2), that guide cell fate decisions during embryonic development. PRC1 forms an array of biochemically distinct canonical PRC1 (cPRC1) or non-canonical PRC1 (ncPRC1) complexes characterized by the mutually exclusive presence of PCGF (PCGF1-PCGF6) paralog subunit; however, whether each one of these subcomplexes fulfills a distinct role remains largely controversial. Here, by performing a CRISPR-based loss-of-function screen in embryonic stem cells (ESCs), we uncovered a previously unappreciated functional redundancy among PRC1 subcomplexes. Disruption of ncPRC1, but not cPRC1, displayed severe defects in ESC pluripotency. Remarkably, coablation of non-canonical and canonical PRC1 in ESCs resulted in exacerbation of the phenotype observed in the non-canonical PRC1-null ESCs, highlighting the importance of functional redundancy among PRC1 subcomplexes. Together, our studies demonstrate that PRC1 subcomplexes act redundantly to silence lineage-specific genes and ensure robust maintenance of ESC identity.
Keywords: PCGF; PRC1; Polycomb; RING1A/B; cPRC1; embryonic stem cells; germ layer lineages; ncPRC1; pluripotency; redundancy.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


Comment in
-
PRC1 uncomplexed.Stem Cell Reports. 2022 May 10;17(5):1009-1011. doi: 10.1016/j.stemcr.2022.04.010. Stem Cell Reports. 2022. PMID: 35545021 Free PMC article.
Similar articles
-
Polycomb complexes redundantly maintain epidermal stem cell identity during development.Genes Dev. 2021 Mar 1;35(5-6):354-366. doi: 10.1101/gad.345363.120. Epub 2021 Feb 18. Genes Dev. 2021. PMID: 33602871 Free PMC article.
-
Pcgf6, a polycomb group protein, regulates mesodermal lineage differentiation in murine ESCs and functions in iPS reprogramming.Stem Cells. 2014 Dec;32(12):3112-25. doi: 10.1002/stem.1826. Stem Cells. 2014. PMID: 25187489
-
Functional Landscape of PCGF Proteins Reveals Both RING1A/B-Dependent-and RING1A/B-Independent-Specific Activities.Mol Cell. 2019 Jun 6;74(5):1037-1052.e7. doi: 10.1016/j.molcel.2019.04.002. Epub 2019 Apr 24. Mol Cell. 2019. PMID: 31029542 Free PMC article.
-
Mammalian PRC1 Complexes: Compositional Complexity and Diverse Molecular Mechanisms.Int J Mol Sci. 2020 Nov 14;21(22):8594. doi: 10.3390/ijms21228594. Int J Mol Sci. 2020. PMID: 33202645 Free PMC article. Review.
-
Post-Embryonic Phase Transitions Mediated by Polycomb Repressive Complexes in Plants.Int J Mol Sci. 2021 Jul 14;22(14):7533. doi: 10.3390/ijms22147533. Int J Mol Sci. 2021. PMID: 34299153 Free PMC article. Review.
Cited by
-
The PRC2.1 subcomplex opposes G1 progression through regulation of CCND1 and CCND2.Elife. 2025 Feb 4;13:RP97577. doi: 10.7554/eLife.97577. Elife. 2025. PMID: 39903505 Free PMC article.
-
USP7 represses lineage differentiation genes in mouse embryonic stem cells by both catalytic and noncatalytic activities.Sci Adv. 2023 May 19;9(20):eade3888. doi: 10.1126/sciadv.ade3888. Epub 2023 May 17. Sci Adv. 2023. PMID: 37196079 Free PMC article.
-
Chromobox protein homolog 7 suppresses the stem-like phenotype of glioblastoma cells by regulating the myosin heavy chain 9-NF-κB signaling pathway.Cell Death Discov. 2025 Feb 23;11(1):74. doi: 10.1038/s41420-025-02362-7. Cell Death Discov. 2025. PMID: 39988672 Free PMC article.
-
Microglial PCGF1 alleviates neuroinflammation associated depressive behavior in adolescent mice.Mol Psychiatry. 2025 Mar;30(3):914-926. doi: 10.1038/s41380-024-02714-2. Epub 2024 Aug 30. Mol Psychiatry. 2025. PMID: 39215186 Free PMC article.
-
[PCGF1 is highly expressed in rectal adenocarcinoma and silencing PCGF1 inhibits proliferation of rectal adenocarcinoma cells in vitro].Nan Fang Yi Ke Da Xue Xue Bao. 2022 Sep 20;42(9):1296-1302. doi: 10.12122/j.issn.1673-4254.2022.09.04. Nan Fang Yi Ke Da Xue Xue Bao. 2022. PMID: 36210701 Free PMC article. Chinese.
References
-
- Akasaka T., van Lohuizen M., van der Lugt N., Mizutani-Koseki Y., Kanno M., Taniguchi M., Vidal M., Alkema M., Berns A., Koseki H. Mice doubly deficient for the Polycomb Group genes Mel18 and Bmi1 reveal synergy and requirement for maintenance but not initiation of Hox gene expression. Development. 2001;128:1587–1597. - PubMed
-
- Boyer L.A., Plath K., Zeitlinger J., Brambrink T., Medeiros L.A., Lee T.I., Levine S.S., Wernig M., Tajonar A., Ray M.K., et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349–353. - PubMed
-
- Coré N., Bel S., Gaunt S.J., Aurrand-Lions M., Pearce J., Fisher A., Djabali M. Altered cellular proliferation and mesoderm patterning in Polycomb-M33-deficient mice. Development. 1997;124:721–729. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

