Neurological sequela and disruption of neuron-glia homeostasis in SARS-CoV-2 infection
- PMID: 35364273
- PMCID: PMC8963977
- DOI: 10.1016/j.nbd.2022.105715
Neurological sequela and disruption of neuron-glia homeostasis in SARS-CoV-2 infection
Abstract
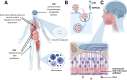
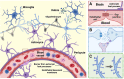
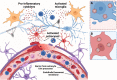
The coronavirus disease 2019 (COVID-19) pandemic is responsible for 267 million infections and over 5 million deaths globally. COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a single-stranded RNA beta-coronavirus, which causes a systemic inflammatory response, multi-organ damage, and respiratory failure requiring intubation in serious cases. SARS-CoV-2 can also trigger neurological conditions and syndromes, which can be long-lasting and potentially irreversible. Since COVID-19 infections continue to mount, the burden of SARS-CoV-2-induced neurologic sequalae will rise in parallel. Therefore, understanding the spectrum of neurological clinical presentations in SARS-CoV-2 is needed to manage COVID-19 patients, facilitate diagnosis, and expedite earlier treatment to improve outcomes. Furthermore, a deeper knowledge of the neurological SARS-CoV-2 pathomechanisms could uncover potential therapeutic targets to prevent or mitigate neurologic damage secondary to COVID-19 infection. Evidence indicates a multifaceted pathology involving viral neurotropism and direct neuroinvasion along with cytokine storm and neuroinflammation leading to nerve injury. Importantly, pathological processes in neural tissue are non-cell autonomous and occur through a concerted breakdown in neuron-glia homeostasis, spanning neuron axonal damage, astrogliosis, microgliosis, and impaired neuron-glia communication. A clearer mechanistic and molecular picture of neurological pathology in SARS-CoV-2 may lead to effective therapies that prevent or mitigate neural damage in patients contracting and developing severe COVID-19 infection.
Keywords: Astrocyte; Axon; COVID-19; Cytokine storm; Extracellular vesicles; Immune system; Microglia; Neuron; Oligodendrocyte.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
MGS, ELF, and AMS declare no conflicts of interest.
Figures
References
-
- Barberis E., Vanella V.V., Falasca M., Caneapero V., Cappellano G., Raineri D., Ghirimoldi M., De Giorgis V., Puricelli C., Vaschetto R., Sainaghi P.P., Bruno S., Sica A., Dianzani U., Rolla R., Chiocchetti A., Cantaluppi V., Baldanzi G., Marengo E., Manfredi M. Circulating exosomes are strongly involved in SARS-CoV-2 infection. Front. Mol. Biosci. 2021;8 - PMC - PubMed
-
- Beyrouti R., Adams M.E., Benjamin L., Cohen H., Farmer S.F., Goh Y.Y., Humphries F., Jäger H.R., Losseff N.A., Perry R.J., Shah S., Simister R.J., Turner D., Chandratheva A., Werring D.J. Characteristics of ischaemic stroke associated with COVID-19. J. Neurol. Neurosurg. Psychiatry. 2020;91:889–891. - PMC - PubMed
-
- Boroujeni M.E., Simani L., Bluyssen H.A.R., Samadikhah H.R., Zamanlui Benisi S., Hassani S., Akbari Dilmaghani N., Fathi M., Vakili K., Mahmoudiasl G.R., Abbaszadeh H.A., Hassani Moghaddam M., Abdollahifar M.A., Aliaghaei A. Inflammatory response leads to neuronal death in human post-mortem cerebral cortex in patients with COVID-19. ACS Chem. Neurosci. 2021;12(12):2143–2150. - PubMed
-
- Brann D.H., Tsukahara T., Weinreb C., Lipovsek M., Van den Berge K., Gong B., Chance R., Macaulay I.C., Chou H.J., Fletcher R.B., Das D., Street K., de Bezieux H.R., Choi Y.G., Risso D., Dudoit S., Purdom E., Mill J., Hachem R.A., Matsunami H., Logan D.W., Goldstein B.J., Grubb M.S., Ngai J., Datta S.R. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci. Adv. 2020;6(31) - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

