Neuroprotective effect of liraglutide in an experimental mouse model of multiple sclerosis: role of AMPK/SIRT1 signaling and NLRP3 inflammasome
- PMID: 35364735
- PMCID: PMC9135867
- DOI: 10.1007/s10787-022-00956-6
Neuroprotective effect of liraglutide in an experimental mouse model of multiple sclerosis: role of AMPK/SIRT1 signaling and NLRP3 inflammasome
Abstract
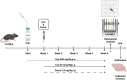
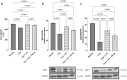
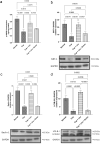
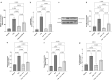
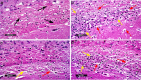
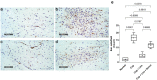
The heterogeneous nature of multiple sclerosis (MS) and the unavailability of treatments addressing its intricate network and reversing the disease state is yet an area that needs to be elucidated. Liraglutide, a glucagon-like peptide-1 analogue, recently exhibited intriguing potential neuroprotective effects. The currents study investigated its potential effect against mouse model of MS and the possible underlying mechanisms. Demyelination was induced in C57Bl/6 mice by cuprizone (400 mg/kg/day p.o.) for 5 weeks. Animals received either liraglutide (25 nmol/kg/day i.p.) or dorsomorphin, an AMPK inhibitor, (2.5 mg/Kg i.p.) 30 min before the liraglutide dose, for 4 weeks (starting from the second week). Liraglutide improved the behavioral profile in cuprizone-treated mice. Furthermore, it induced the re-myelination process through stimulating oligodendrocyte progenitor cells differentiation via Olig2 transcription activation, reflected by increased myelin basic protein and myelinated nerve fiber percentage. Liraglutide elevated the protein content of p-AMPK and SIRT1, in addition to the autophagy proteins Beclin-1 and LC3B. Liraglutide halted cellular damage as manifested by reduced HMGB1 protein and consequently TLR-4 downregulation, coupled with a decrease in NF-κB. Liraglutide also suppressed NLRP3 transcription. Dorsomorphin pre-administration indicated a possible interplay between AMPK/SIRT1 and NLRP3 inflammasome activation as it partially reversed liraglutide's effects. Immunohistochemical examination of Iba+ microglia emphasized these findings. In conclusion, liraglutide exerts neuroprotection against cuprizone-induced demyelination via anti-inflammatory, autophagic flux activation, NLRP3 inflammasome suppression, and anti-apoptotic mechanisms, possibly mediated, at least in part, via AMPK/SIRT1, autophagy, TLR-4/ NF-κB/NLRP3 signaling. The potential mechanistic insight of Lira in alleviating Cup-induced neurotoxicity via: (1) AMPK/SIRT1 pathways activation resulting in the stimulation of brain autophagy flux (confirmed by lowering Beclin-1 and LC3-B protein expression). (2) Inhibition of NLRP3 inflammasome activation, as evidenced by reduced HMGB1, TLR-4, NF-κB and NLRP3 protein expression, alongside diminishing the activation of its downstream cascade as reflected by reduced levels of caspase-1 and IL-1β protein expression. (3) A possible modulating interplay between the previously mentioned two pathways.
Keywords: AMPK/SIRT1; Autophagy; Dorsomorphin; Liraglutide; Multiple sclerosis; NLRP3 inflammasome.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
