Mechanisms of neuroinflammation in hydrocephalus after intraventricular hemorrhage: a review
- PMID: 35365172
- PMCID: PMC8973639
- DOI: 10.1186/s12987-022-00324-0
Mechanisms of neuroinflammation in hydrocephalus after intraventricular hemorrhage: a review
Abstract
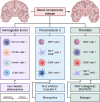
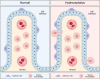
Intraventricular hemorrhage (IVH) is a significant cause of morbidity and mortality in both neonatal and adult populations. IVH not only causes immediate damage to surrounding structures by way of mass effect and elevated intracranial pressure; the subsequent inflammation causes additional brain injury and edema. Of those neonates who experience severe IVH, 25-30% will go on to develop post-hemorrhagic hydrocephalus (PHH). PHH places neonates and adults at risk for white matter injury, seizures, and death. Unfortunately, the molecular determinants of PHH are not well understood. Within the past decade an emphasis has been placed on neuroinflammation in IVH and PHH. More information has come to light regarding inflammation-induced fibrosis and cerebrospinal fluid hypersecretion in response to IVH. The aim of this review is to discuss the role of neuroinflammation involving clot-derived neuroinflammatory factors including hemoglobin/iron, peroxiredoxin-2 and thrombin, as well as macrophages/microglia, cytokines and complement in the development of PHH. Understanding the mechanisms of neuroinflammation after IVH may highlight potential novel therapeutic targets for PHH.
Keywords: Complement; Intraventricular hemorrhage; Macrophages; Microglia; Neuroinflammation; Posthemorrhagic hydrocephalus.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Christian EA, Jin DL, Attenello F, Wen T, Cen S, Mack WJ, et al. Trends in hospitalization of preterm infants with intraventricular hemorrhage and hydrocephalus in the United States, 2000–2010. J Neurosurg Pediatr. 2016;17(3):260–269. - PubMed
-
- Darby DG, Donnan GA, Saling MA, Walsh KW, Bladin PF. Primary intraventricular hemorrhage: clinical and neuropsychological findings in a prospective stroke series. Neurology. 1988;38(1):68–75. - PubMed
-
- Zhang S, Jia B, Li H, You C, Hanley DF, Jiang Y. Primary intraventricular hemorrhage in adults: etiological causes and prognostic factors in Chinese population. J Neurol. 2017;264(2):382–390. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

